Nanotechnology has emerged as an exciting and promising new means of treating neurological disease. CD Bioparticles specializes in targeted drug delivery to the central nervous system (CNS), utilizing nano-carriers and viral vectors for optimal bioavailability and efficacy. Our services include design, loading, characterization, off-target evaluation, and activity testing. We offer custom design of delivery systems, as well as formulation development and optimization of dosing regimens. CD Bioparticles' experienced team of scientists is dedicated to providing high-quality services in the field of CNS drug delivery.
Neurodegenerative diseases, including the two most common ones - Alzheimer's disease (AD) and Parkinson's disease (PD), significantly impact the lives of over 6 million Americans, highlighting the importance of effective treatments for central nervous system (CNS) disorders[1]. Other vital areas of CNS disorder treatment include neuroregeneration, brain tumors, spinal cord injury, and others, emphasizing the critical need for continuous research and development in this field. The key barrier to drug delivery to the brain is the blood-brain barrier (BBB), typically formed by tight brain endothelium and surrounded by basal and neurovascular cells such as pericytes and astrocytes. This tight vascular interface in the brain generally imparts low transcellular permeability to most molecules, thereby maintaining brain function. However, this characteristic of the blood-brain barrier in the central nervous system also hinders entry of most therapeutic drugs to the central nervous system, thereby impeding treatment of many central nervous system disorders.
To deliver drugs to the central nervous system (CNS), CNS-targeted drug delivery systems have been developed and designed with the following characteristics:
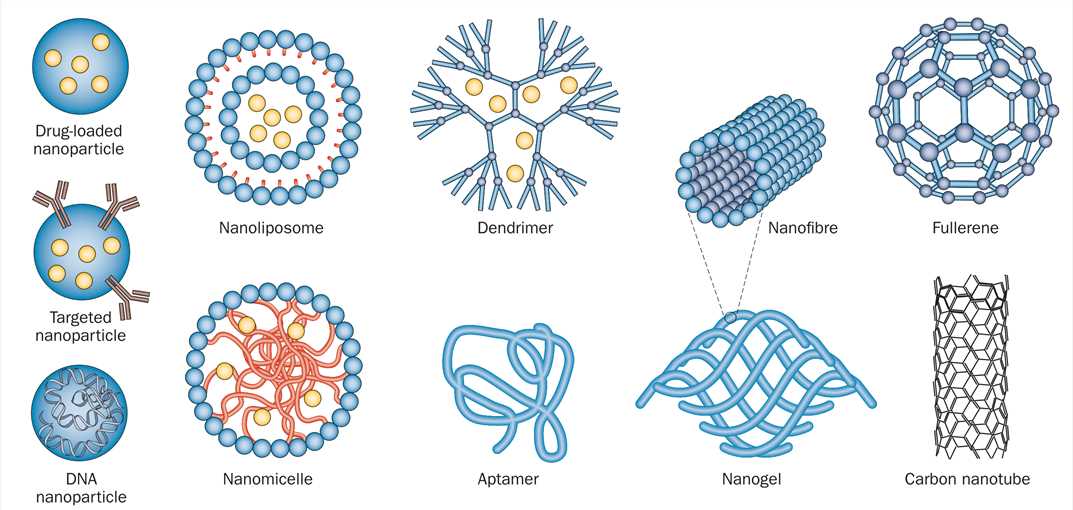
Figure 1 Schematic representation of nanoparticles that have been applied as therapies for CNS disease[1].
Many different forms of nanotechnology exist, each of which provides unique properties that can be utilized for CNS therapeutics. AAV vector, HSV vector, rabies virus vector and other CNS tropism virus vectors are also widely used as drug delivery carriers with central nervous system[2]. Below is a partial list of FDA-approved CNS-targeting drugs for your reference.
Table 1 FDA-approved CNS-targeting drugs
| Name | Ingredient active | Carrier | Indication | Date of Approval |
|---|---|---|---|---|
| Avinza® (Pfizer) | Morphine sulfate | Nanocrystals | Mental stimulant | FDA (2002/2015) |
| Ritalin LA® (Novartis) | Methylphenidate HCl | Nanocrystals | Mental stimulant | FDA (2002) |
| Focalin XR® (Novartis) | Dexmethylphenidate HCl | Nanocrystals | Mental stimulant | FDA (2005) |
| Invega® (Janssen Pharmaceuticals) | Paliperidone | Nanocrystal | Schizophrenia | FDA (2009) |
| Invega Sustenna® (Janssen Pharmaceuticals) | Paliperidone Palmitate | Nanocrystal | Schizophrenia | FDA (2009) |
| Invega® Sustenna® (Janssen Pharms) | Paliperidone palmitate | Nanocrystals | Schizophrenia schizoaffective disorder | FDA (2009/2014) |
| Nanotherm® (MagForce) | Iron oxide | Aminosilane-coated Iron nanoparticles | Brain tumor | FDA (2010) |
| Rykindo® | Risperidone | Microspheres | Schizophrenia | FDA (2023) |
References:
1. Srikanth M, Kessler JA: Nanotechnology-novel therapeutics for CNS disorders. Nat Rev Neurol. 2012, 8(6):307-318.
2. Deverman BE, Ravina BM, Bankiewicz KS, Paul SM, Sah DWY: Gene therapy for neurological disorders: progress and prospects. Nat Rev Drug Discov. 2018, 17(9):641-659.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.