CD Bioparticles offers a comprehensive platform for the design, synthesis, ligand conjugation, modification, and evaluation of drug delivery carriers based on nano-carriers for Gastrointestinal tract targeting. Our professional carrier construction, combined with in vivo and in vitro analysis and characterization, enables us to provide a range of products and services that meet the unique needs of Gastrointestinal tract targeted drug delivery systems. We provide full support for early development, leveraging the specific characteristics of the Gastrointestinal tract to ensure optimal efficacy.
The gastrointestinal (GI) tract is composed of four major compartments that play a critical role in drug release and absorption: the mouth, stomach, small intestine, and large intestine. Each section of the GI tract has distinct biochemical, acidic, fluid, and enzyme characteristics that pose targeting challenges for orally administered drugs. In Figure 1, the pH characteristics of the gastrointestinal tract are presented (alongside the controlled release attributes of a typical pH-responsive material). When drugs have poor solubility, stability, and bioavailability, treating diseases through oral administration can be especially difficult. Various approaches have been explored to overcome these limitations, with the nano-delivery system gaining particular attention. Drug-loaded nano-carriers such as polymeric nanoparticles, lipid nanoparticles, inorganic nanoparticles, micelles, nano-emulsions, and liposomes have been developed to improve drug dispersion, solubility, and stability. These methods also offer potential for gastrointestinal absorption of peptide drugs and vaccines. Local treatments for gastrointestinal diseases like gastritis, Crohn's disease, gastrointestinal cancer, and systemic diseases like diabetes, neurological disease, and vaccines require efficient drug delivery systems to overcome the gastrointestinal biological barrier and enhance local targeting[1].
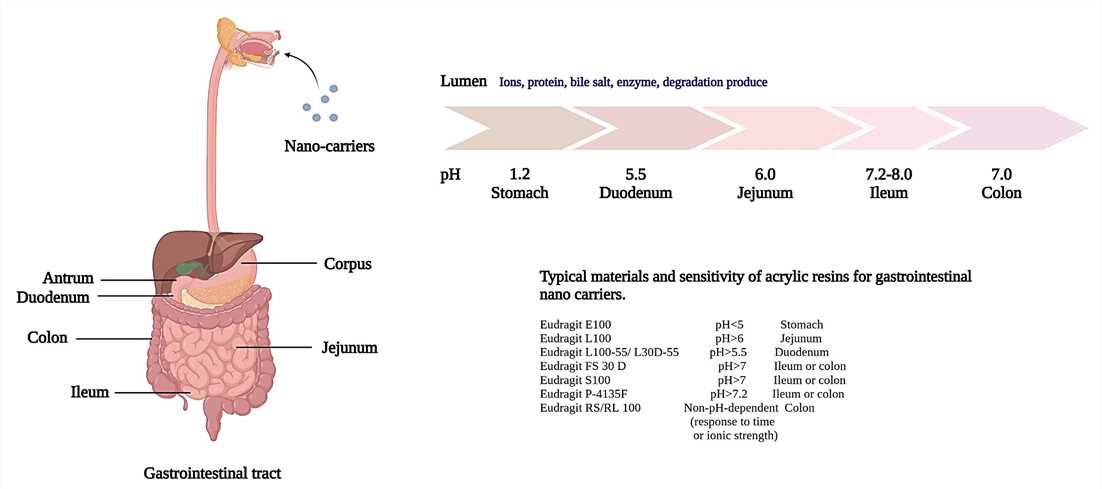 Figure 1. Schematic model of gastrointestinal tract. Each segment has different pH values and epithelial characteristics.
Figure 1. Schematic model of gastrointestinal tract. Each segment has different pH values and epithelial characteristics.
Different drugs target different sites based on the physiological characteristics of those sites. Table 1 below highlights some of these features:
Table 1. Features for gastrointestinal response.
| Gastrointestinal parts | Features | Function |
|---|---|---|
| Gastric response | Acid resistant (stability), slow release (long duration of action), less irritating to the gastric mucosa, not easily cleared by the stomach | Prolong gastric retention time and release drugs continuously to improve local treatment |
| Small intestinal response | Acid resistance, high bioavailability and stability, so as not to be destroyed by digestive juices such as stomach acid and bile. Targeted (targeted action on small intestinal tissue) |
Intestinal absorption of biodegradable drugs; Absorption of drugs for systemic diseases; Deliver drugs to the appropriate intestinal absorption site to reduce dose and improve safety. |
| Colon response | Intestinal flora can be used, such as the use of biodegradable materials; targeting and specificity, biocompatibility and low toxicity (does not affect the intestinal flora) |
Sustained release and slow absorption of gastrointestinal degradable drugs; Delivery of local therapeutic drugs to the colon. |
| Mucus response | Remain in mucus or penetrate mucus, control the slow release of drugs, and target specific factors. | Enhance retention time and the local drug concentration to improve local treatment or systemic absorption. Or reach the epithelial intactly and avoid being cleared rapidly to improve local treatment or systemic absorption. |
Targeted drug delivery systems have been developed to improve the efficiency and efficacy of drug delivery in different parts of the gastrointestinal tract.
All of these targeted drug delivery systems have the potential to improve the efficacy and safety of drug treatments for various diseases[2].
Table 2 Typical materials for the nano-carriers
| Category | Typical Carrier-surface Material / Decoration | Main mechanism | |
|---|---|---|---|
| Mucus adhesive | Synthetic polymer | Poly (lactic acid) (PLA), Poly (sebacic acid) (PSA), Poly (lacticacid-glycolic acid) (PLGA), Poly (acrylic acid) (PAA), Carbopol, Polycarbophil, Copolymer of methylvinylether and methacrylic acid, and their Derivatives | Hydrogen bonding, Electrostatic interaction, Long-chain entanglement |
| Cellulose derivative | Carboxy methyl cellulose (CMC), Thiolated carboxy methyl cellulose (TCMC), Sodium carboxy methyl cellulose (SCMC), Hydroxyethyl cellulose (HEC), Hydroxypropyl cellulose (HPC), Methyl hydroxyethyl cellulose (MHPC), Methyl cellulose (MC), Hydroxypropyl methyl- cellulose (HPMC) | Hydrogen bonding, Electrostatic interaction, Long-chain entanglement | |
| Natural polysaccharide | Chitosan, Gelatin, Hyaluronic acid, Carrageenan, Pectin, Sodium alginate and their derivatives | Hydrogen bonding, Electrostatic interaction, Long-chain entanglement | |
| Natural protein | Keratose, Kerateine | Hydrogen bonding, Electrostatic interaction, Covalent disulfide bond | |
| Sulfhydrylation agent | Cysteine, Thioglycolic Acid (TGA), 4-Thiobuthylamidine (TBA), N-acetyl-cysteine, Isopropyl-S-acetyl thioacetimidate, Glutathione | Covalent disulfide bond | |
| Mucus penetration | Synthetic polymer | Polyethylene glycol (PEG), Pluronic F127 (PF127), Poly(2-hydroxypropyl) methacrylamide (PHPMA), Polysarcosine (PSAR), Poly(vinyl alcohol) (PVA), Poly(2-alkyl-2-oxazoline) (PAOXA), Hydroxyl-containing non-ionic water-soluble polymers, Zwitterionic polymers (polybetaines) | Shielding electrostatic and hydrophobic interactions |
| Small molecular compound | N-acetylcysteine, 2-mercapto-N-octylacetamide, N-dodecyl-4-mercaptobutylamide | Destroying mucin disulfide bond | |
| Proteolytic enzyme | Papain, Bromelain, Trypsin | Hydrolyzed mucin | |
| Promote transcellular transport |
Penetration enhancer |
Nonionic surfactants (such as Pluronic P123, F68 and F127) |
Alter cell membrane fluidity Decrease transepithelial resistance; Inhibit the effect of P-glycoprotein efflux |
| Bile salts and derivatives | Alter cell membrane fluidity; Interact with bile acid transporter to induce endocytosis and uptake | ||
| Medium-chain fatty acids and derivatives | Alter cell membrane fluidity; Decrease transepithelial resistance | ||
|
Positively charged polymer |
Chitosan and derivatives | Reversible decrease of the transepithelial resistance; Electrostatic interaction with cell surface prolongs absorption time | |
|
Cell penetrating peptide |
HIV-1 Tat, penetratin, oligoarginine, MAP and R8 | Assist carriers enter directly into cells through lipid membrane or be internalized by endocytosis. | |
| Broad enterocyte surface targeting ligand | IgG (for Neonatal Fc receptor (FcRn, FCGRT)); Lactoferrin (for Lactoferrin receptor (ITLN-1)); Vitamin B12 (for Vitamin B12–intrinsic factor receptor (CUBN, AMN)) | Trigger transcytosis mechanism of receptors in cell membrane | |
| M cell targeting ligand | Lectin (UEA-1, WGA, TL and LTA) | Combine with cell surface glycoproteins and sugar esters to prolong absorption and promote endocytosis | |
| HA recombinant proteins whose C terminus are introduced with CPE30 (the terminal 30 amino acids of Clostridium Perfringens enterotoxin) | Bound to claudin-4 receptor that is highly expressed in M cells | ||
| RGD and LDV peptidomimetics |
Bound to integrins on the apical surface of M cells |
||
| Promote paracellular transport |
Penetration enhancer |
Nonionic surfactants, bile salts, medium-chain fatty acids and their derivatives | Irreversibly dissolve cell membrane components Combine with extracellular Ca2+ to induce redistribution of target proteins of calmodulin |
|
Positively charged polymer |
Chitosan and derivatives | Possible mechanism is reversible interaction with tight junction proteins | |
| Peptide | ADT-6 HAV-6 C-CPE 7-mer(FDFWITP or PN-78) AT-1002 PN159(KLAL or MAP) | Interacting with occludin and claudins to regulate the tight junction opening | |
| Myosin light chain phosphatase (MLCP) inhibitory peptides 640 and 250 (synthetic peptides emulating interfacial contacts involving MLCP regulatory proteins CPI-17 and MYPT) | Inhibit dephosphorylation catalyzed by MLCP to prevent reversion of opened tight junction | ||
| Bacterial protein | Clostridium perfringens enterotoxin (CPE) | Binding to claudins to regulate the tight junction opening | |
| Metal chelating agent | Citrate | Combine with extracellular Ca to induce redistribution of target proteins of calmodulin |
Quotations and Ordering

References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.