Nanoparticles for Neurological Disease Treatment
CD Bioparticles is a leading manufacturer and supplier of various drug delivery products, including metal nanostructures, biomacromolecules, synthetic polymer and biopolymers and lipid system, for R&D and commercialization in a variety of application areas. We also have developed mature technology platforms for drug delivery, such as inorganic nanomaterials, biomacromolecules, polymeric and lipid system. In addition, we can offer a wide range of custom services including drug delivery nanoparticles formulation, bioparticles analysis and characterization, and drug targeting strategy. We are dedicated to providing the most comprehensive list of products and fit-for-purpose custom analysis and synthesis services to academia as well as industrial researchers and assay developers all around the world.
Polymeric nanoparticles (NPs) are most commonly used nano-delivery vehicles for neurological disease treatment due to their ability to cross the cellular tight junctions, bypass the blood-brain barrier (BBB), encapsulate higher drug content and release site-specific drug after being conjugated to ligands. The size of these polymeric NPs ranges from 10 nm to 1000nm and they possess the following advantages: controllable drug release pattern, higher bioavailability, flexible modification to achieve targeted delivery by attaching suitable ligands to their surface.
Nanoparticles in Neurological Disease Treatment
There is an increasing incidence of neurological diseases (e.g. Alzheimer's disease (AD), Parkinson's disease (PD), multiple sclerosis (MS), and primary brain tumors) among people, especially in the aging population. Since these diseases are of the central nervous system (CNS), BBB presents an impediment for their treatment and diagnosis. Only lipophilic molecules or molecules below 400-600 Da in weight can cross the BBB, which limits some potential therapeutic and diagnostic tools. As a result, to overcome the above issue caused by the BBB, nanobiotechnology, applying nanoscale objects (< 100 nm) to pharmaceutical and biomedical systems is attracting more interests due to its alterable properties of materials (e.g. nanocapsules, nanospheres, polymeric nanosuspensions, polymeric nanogels, polymeric nanomicelles and polymeric nanoliposomes) at that size. In particular, nanoparticle delivery systems could provide alternate ways for targeted drug delivery to the CNS and for new therapeutic applications. Moreover, since NPs are able to traverse the BBB, they offer potential opportunities for the diagnosis of CNS diseases at the early stage and for monitoring and detecting further disease progression.
NPs are applied as carrier systems to assist in targeting the CNS drug. Generally, there are two methods to conjugate a drug to an NP: 1) incorporate drugs during NP production; 2) incorporate drugs by surface adsorption onto the preformed NP. Drugs conjugated to NPs may escape from the degradation and phagocytosis of the reticuloendothelial system. In addition, these drugs possess a more sustained releasing pattern which means a slow release of drugs. There are several ways by which solutes move across the BBB and diffusion is one of them, which is a very simple passive process. In this way, drugs can be transported under the effect of the concentration gradient existing across the membranes or between the cells. The process of diffusion depends on size and lipophilicity of the solutes. The other way is carrier-mediated transport. In this way, drugs will be transported by active or passive processes. In eukaryotic cells, to selective uptake of macromolecules across the BBB is achieved by the receptor-mediated endocytosis and endogenous peptides are ideal for therapeutic delivery into neuronal cells through this process. For example, locked nucleic acids (LNAs) conjugated with NPs are of potential significance because they enhance the delivery of NPs and display a therapeutic effectiveness (Figure 1).
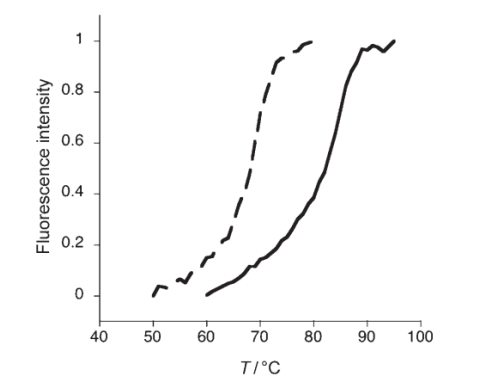
Figure 1. Normalized fluorescence melting curves of NP conjugates after incubation with fluorescein labeled complementary oligonucleotides. A high Tm (85±1ºC) was observed for the LNP–complementary oligonucleotide complex (solid curve) as compared to the DNP–complementary oligonucleotide complex (Tm= 69m±1ºC broken curve). (Seferos, D. S., Giljohann, D. A., Rosi, N. L., & Mirkin, C. A. (2007). Locked Nucleic Acid–Nanoparticle Conjugates. ChemBioChem, 8(11), 1230–1232)
However, to be effective after NP drug delivery to the CNS, the issue of nonspecific binding must be surmounted. Researchers usually functionalize the NPs by surface modification and conjugation with transporters of the BBB to solve this problem. Vascular endothelial growth factor, epidermal growth factor, insulin-like growth factor, insulin, albumin, transferrin, TAT-derived peptides, polyarginines, OX 26, MAb 8D3, RI7217, and lactoferrin (Lf) are commonly used to conjugate with NPs to improve targeting across BBB. For example, Hu, et al. found that NPs conjugated with Lf were with greater BBB absorption than unconjugated NPs due to the presence of the Lf receptor on the surface of the BBB (Table 1).
Table 1. Pharmacokinetic parameters of coumarin-6 in brain and whole blood following injection of coumarin-6 loaded NP or Lf–NP in mice.
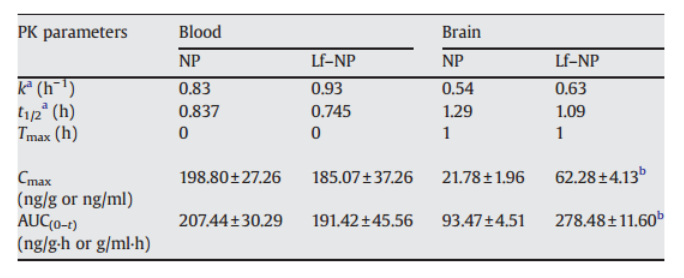
a: k and t1/2 denotes elimination rate constant and elimination half life respectively. b: Mean±S.D. Significantly different from NP, p<0.05. Lf–NP: Lf conjugated nanoparticle. (Hu, K., Li, J., Shen, Y., Lu, W., Gao, X., Zhang, Q., & Jiang, X. (2009). Lactoferrin-conjugated PEG–PLA nanoparticles with improved brain delivery: In vitro and in vivo evaluations. Journal of Controlled Release, 134(1), 55–61)
Exosomes in Neurological Disease Treatment
Exosomes are nanometer-sized membrane vesicles secreted by a variety of cell types and can be isolated from conditioned cell culture media or bodily fluids such as urine and plasma. Once released from a cell, exosomes can fuse with the membrane of another cell, transferring exosomal molecules from one cell to another. This property provides exosomes with the opportunity to transport drugs into cells. The uptake mechanism of exosomes is clathrin-mediated endocytosis followed by reverse fusion with the restricting membrane of endosomes, thus making it possible for exosomes to cross the intact blood-brain barrier.
Exosomes, which are released by living cells, constitute the body's natural delivery system. Exosomes are superior to existing therapies due to their minimal immunological reaction, absence of toxicity, and enhanced circulatory system stability. Autologous dendritic cells were used by Alvarez-Erviti and colleagues to produce exosomes. The targeting peptides of brain and muscle functionalizes these exosomes. The results that exosomes specifically deliver siRNA to brain neurons, microglia, and oligodendrocytes, resulting in targeted gene silencing after intravenous administration.
Liposomes in Neurological Disease Treatment
Liposomes are spherical vesicles composed of a phospholipid bilayer. They have both a hydrophilic core and a hydrophobic lipid bilayer. Therefore, various active substances can be loaded onto liposomes. In addition, depending on the composition of the formulation, properties such as liposome size, zeta potential, and rigidity can be controlled. The surface of liposomes can be modified for different purposes. Among them, the specific targeting of liposomes can be achieved by conjugating appropriate targeting carriers. For drug delivery to the brain, cationic liposomes need to be used to facilitate crossing the blood-brain barrier through adsorption-mediated transcytosis. In recent years, many liposomal DDS have been established to treat AD, such as:
Rivastigmine is one of the FDA-approved drugs for the treatment of AD, but its plasma elimination half-life is very short, about 1.5 hours. And the study found that its brain permeability is limited by tight junctions. Encapsulation of rivastigmine in dipalmitoylphosphatidylcholine (DPPC)/cholesterol liposomes increased drug delivery of the brain when administered orally and intraperitoneally to mice.
Metal Nanoparticlesin Neurological Disease Treatment
Compared with polymeric nanoparticles and lipid carriers, research on the use of metal nanoparticles for drug delivery in the brain is limited. Metal nanoparticles range in size from 1 to 200 nanometers and, depending on their size and shape, exhibit specific physicochemical properties. Metal nanoparticles include different types such as gold, silver, and titanium dioxide. It has been reported that transferrin-modified gold nanoparticles (AuNPs) and gold nanorods (AuNRs) can cross the blood-brain barrier in vivo and in vitro. Under the irradiation of a certain concentration of near-infrared light (NIR), the blood-brain barrier can be opened without significantly reducing cell viability, and the gold nano-preparation can preferentially accumulate in the neurogenic region of the brain of the mouse model. These results provide direction for the development of gold-based drug delivery system (DDS), combined with transferrin peptide and laser activation technology, for the treatment of neurodegenerative diseases. The researchers also developed a gold nanoparticle-based multifunctional anti-Aβ (amyloid beta) drug. First, polyoxometalates with Wells-Dawson structure were grafted on the surface of gold nanoparticles, which were then conjugated with LPFFD peptide to be used as β-inhibitors. The results of the study showed that it can synergistically inhibit Aβ aggregation, dissociate Aβ fibrils, reduce Aβ-mediated peroxidase activity and reduce Aβ-induced cytotoxicity in vitro. The drug delivery system was able to cross the blood-brain barrier in mouse models when administered via tail vein injection.
Quantum dots in Neurological Disease Treatment
Quantum dots are a type of colloidal semiconductor nanocrystal composed of a metalloid crystalline core (such as cadmium selenium) surrounded by an intermediate layer with a non-reactive metal shell (such as zinc sulfide). This inorganic nanomaterial has high brightness, long-term photostability, and narrow emission spectrum with tunable size, so it has a wide range of applications in diagnostic fields, such as revolutionary imaging technology, namely fluorescent probes. Based on their high surface area properties, quantum dots have the potential to combine a variety of diagnostic and therapeutic molecules for targeted treatment of central nervous system diseases, so they are considered to be promising carriers for drug delivery to the blood-brain barrier. By chemically modifying the outer layer of quantum dots with biologically active molecules, their water solubility and desired biological activities can be promoted, thereby achieving directional targeting of specific molecules while carrying therapeutic molecules.
After intraperitoneal injection of QDs-cap (Captopril-conjugated CdSe/ZnS core/shell quantum dots) in mice, these quantum dots were transported to the brain, liver, spleen and kidney through the systemic blood circulation, which showed that these quantum dots may have the potential to cross the blood-brain barrier. As well known, the Transferrin (Tf) receptor is a specific BBB transporter that allows specific biomolecules to cross the BBB. In a study, by introducing a lysine coating on CdSe/CdS/ZnS quantum dots and then conjugating with Tf, it was shown that after systemic administration, the penetration rate of these Tf-conjugated QDs in an in vitro blood-brain barrier model was concentration- and time-dependent, which validated the receptor-mediated transport mechanism.
In addition to the above advantages, there are still several challenges when developing general and NPs-based drugs for neurological diseases:
-
The candidate NP-based therapeutic agents pass across the BBB in a selective way;
-
Drugs must be maintained at a therapeutic level and should not be rapidly degraded after delivered to the CNS or brain;
-
The NP-based drugs should be effective at a relatively low concentration;
-
Toxicity must be considered when applying a nano-enabled drug delivery system;
-
NPs may induce unexpected immune responses;
-
The organ may present different behavior due to the adaption of tissue cells to NPs.
Our Featured Services
CD Bioparticles is specialized in the development of drug delivery systems and customizing nanocarriers for drug delivery utilizing our core technologies. With our high-quality products and services, the efficacy of your drug delivery can be tremendously improved.
We offer custom synthesis of polymer microspheres and nanoparticles for neurological disease treatment. Clients may select the material type, particle size, size distribution, color dye, fluorescent dye, and/or surface functional groups such as carboxyl or amine groups. We enable you to make wise decisions on all components of the antibody-drug conjugate (ADC) structure so that the optimal candidate is identified for your target of interest in terms of stability, off-target toxicity, and efficacy. Besides, methodologies developed to produce the ADC are simple, scalable and reproducible and ultimately suitable for large-scale manufacture.
References
- Kanwar, J. R., Sun, X., Punj, V., Sriramoju, B., Mohan, R. R., Zhou, S.-F., … Kanwar, R. K. (2012). Nanoparticles in the treatment and diagnosis of neurological disorders: untamed dragon with fire power to heal. Nanomedicine: Nanotechnology, Biology and Medicine, 8(4), 399–414.
- Fornaguera, C., & Solans, C. (2016). Polymeric Nanoparticles for Drug Delivery in Neurological Diseases. Current Pathobiology Reports, 4(4), 189–197.
- Sriramoju, B., K Kanwar, R., & R Kanwar, J. (2014). Nanomedicine based nanoparticles for neurological disorders. Current medicinal chemistry, 21(36), 4154-4168.
- Wong KH, et al.; Review of Current Strategies for Delivering Alzheimer's Disease Drugs across the Blood-Brain Barrier. Int J Mol Sci. 2019, 20(2):381.
- Li X, et al.; Nano carriers for drug transport across the blood-brain barrier. J Drug Target. 2017, 25(1):17-28.



