Bioparticles Penetration and Permeation Test
Highlighted by platforms of bioparticles analysis and characterization, CD Bioparticles provides several methods to determine the drug penetration in order to access drug bioavailability and effectiveness.
Penetration and Permeation Test Introduction
Penetration can occur into the membrane without necessarily diffusing, or passing through, the membrane. Permeation is the movement of the permeant through the membrane that encompasses first partitioning the membrane and then diffusion through the membrane. Penetration and permeation tests are used to profile the permeation behavior of pharmaceutical compounds that can cross cell membranes by passive diffusion, active transport, and pinocytosis to reach the systemic circulation. Penetration and permeability can depend on a variety of factors, such as pH, hydrophilicity and other physico-chemical properties of drugs, and tissue species. As bioparticles are formulated to increase drug penetration and absorption, especially for transdermal drugs, it is important to measure their penetration and permeation abilities to assess drug effectiveness.
Penetration and Permeation Test Methods
Dermal Absorption Test
Dermal (percutaneous, skin) absorption testing is conducted to determine how much drugs penetrates the skin, and thereby whether it has the potential to be absorbed into the systemic circulation. It is considered to occur by passive diffusion with potential affecting factors including drug concentration, exposure period, product formulation, site of exposure, exposure area, and dosage. Assess skin absorption level is conducted to improve the effectiveness of drugs and reduce skin irritation, corrosion, and sensitization. Our team can investigate the dermal penetration by using human, rat or rabbit skin as models for static diffusion and flow-through diffusion testing.
Parallel Artificial Membrane Permeability Assay (PAMPA)
The parallel artificial membrane permeability assay is a recent procedure developed for a rapid determination a substance from a donor compartment through an artificial phospholipid membrane into an acceptor compartment. The main objective is the classification of passively transported compounds via a simple and robust in vitro model of passive transcellular permeation. The ability of this assay to evaluate permeability over a large pH range is valuable for an early understanding of how new oral compounds might be absorbed across the entire gastrointestinal tract. Our team offers a cost-effective method with high reliability and accuracy to predict drug bioavailability.
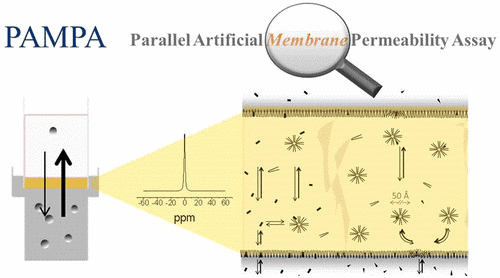
Figure 1. Schematic of the PAMPA model (Mol. Pharmaceutics 2017, 14, 1, 284-295)
Caco-2 Permeability
The Caco-2 permeability assay has been considered to be the golden standard method in nutraceutical and pharmaceutical industries for in vitro prediction of in vivo human intestinal bioabsorption and biouptake of food/nutritional products or medicine candidates. The Caco-2 monolayer is an in vitro model of the human small intestinal mucosa to predict the absorption of orally administered drugs. Caco-2 cells differentiate to form tight junctions between cells to serve as a model of the paracellular movement of compounds across the monolayer when they are cultured as a monolayer. In addition, Caco-2 cells express transporter proteins, efflux proteins, and Phase II conjugation enzymes to model a variety of transcellular pathways as well as the metabolic transformation of test substances. Caco-2 permeability assay measures the rate of transporting of a compound across the Caco-2 cell in both directions. Our team offers custom-design assay to predict human intestinal permeability and to investigate drug efflux in a model that is more similar to in vivo circumstances.
CD Bioparticles offers a full set of service for penetration and permeation tests when drugs are encapsulated into bioparticles. For more detailed information, please feel free to contact us or directly send us an inquiry.
Quotations and Ordering

References:
1. Abd, E., Roberts, M. S., Grice, J. E. A comparison of the penetration and permeation of caffeine into and through human epidermis after application in various vesicle formulations. Skin pharmacology and physiology. 2016, 29(1): 24-30.
2. Ottaviani, G., Martel, S., Carrupt, P. A. Parallel Artificial Membrane Permeability Assay: A New Membrane for the Fast Prediction of Passive Human Skin Permeability. Med. Chem. 2006, 49, 3948.



