CD Bioparticles is a leading manufacturer and supplier of various drug delivery products, including metal nanostructures, biomacromolecules, synthetic polymer and biopolymers and lipid system, for R&D and commercialization in a variety of application areas. We also have developed mature technology platforms for drug delivery, such as inorganic nanomaterials, biomacromolecules, polymeric and lipid system. In addition, we can offer a wide range of custom services including drug delivery nanoparticles formulation, bioparticles analysis and characterization, and drug targeting strategy. We are dedicated to providing the most comprehensive list of products and fit-for-purpose custom analysis and synthesis services to academia as well as industrial researchers and assay developers all around the world.
Nanoparticles (NPs) contribute to medicine in many aspects such as providing a new range of tools and techniques for early detection, disease diagnosis, non-invasive treatment, and disease prevention, which could offer sensitive, rapid and cost-effective solutions for the modern clinical laboratory. To be specific, the application of nanoparticles to medical diagnostics include fluorescent biological labels for imaging, detection of pathogens and proteins, tissue engineering and regeneration, and magnetic resonance imaging (MRI) contrast enhancement.
NPs are being increasingly used in molecular diagnostics and several new technologies are in development as well. Particularly, gold (Au) NPs and quantum dots (QDs) are the most widely used in clinical applications as well as nanobiosensors. Semiconductor QDs are a kind of NPs, which are intense and have stable fluorescence. As a result, they are able to detect tens to hundreds of cancer biomarkers in blood assays or on cancer tissue biopsies at pg/mL concentrations.
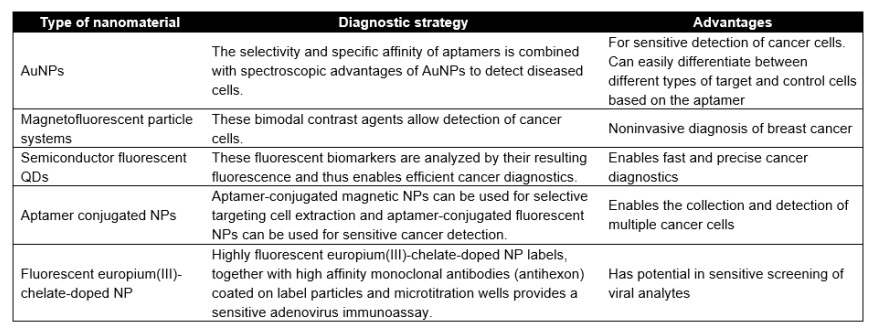
Since they can increase sensitivity and specificity, multiplexing capability and short turnaround times, AuNPs have made great contributions to the molecular diagnostics. In point-of-care testing assays, AuNP-based colorimetric assays show great potential as well, especially the use of AuNP labels in diagnostics and detection. This is because of a unique combination of chemical and physical properties which make it possible to detect biological molecules at low concentrations. In addition, aptamer-conjugated AuNP which is used to collect and detect multiple cancer cells, is another powerful tool for point-of-care diagnostics. Thus, there is a high demand for simple, rapid, efficient and user-friendly alternative methods for the detection of cells, especially for the detection of cancer cells. Cancer is a life-threatening disease; however, the early and accurate detection of cancer is often a bottleneck, which causes its delayed treatment complications. To address these limitations, numerous NPs are under development for effective diagnostics (Table 1).
Table 1. NPs as diagnostic agents.
The development of the effective carrier system means the positive confirmation of the site-specific delivery of the drug. As a result, it is vital to improve the ability for tracking and imaging the fate of any nanomedicine from the systemic to the subcellular level. NPs are ideal for improving the utility of fluorescent markers for medical imaging and diagnostic purposes. However, current techniques have several disadvantages for the application of fluorescent markers in research and clinical diagnostics, including the requirement of color-matched lasers, fluorescence bleaching and lack of discriminatory capacity of multiple dyes, etc. Fluorescent NPs can greatly overcome these problems, especially for imaging tumors and other diseases in vivo.
Fluorescent silica NPs (FSNPs), consisting of silica NPs loaded with fluorescent dye, are a new kind of engineered optical probes. They have gained numerous attentions in cancer imaging. In addition to FSNPs, water-soluble and functionalized QDs are also one of the fastest growing fields of nanotechnology, because they are highly stable against oxidation in biological and biomedical applications. QDs possess stable fluorescent properties and promise a better in vivo imaging and diagnostics for live cells (Figure 1). Magnetic iron oxide NPs with noninvasiveness and low toxicity, are capable of deep-tissue imaging, and are attracting extensive interest as novel contrast agents. Dynamic magnetomotion of magnetic NPs (MNPs) detected with magnetomotive optical coherence tomography (MM-OCT) also represents a new methodology for contrast enhancement and therapeutic interventions in molecular imaging. As a result, various types of nanoparticulate systems are efficiently used as in vitro and in vivo imaging agents, which are ideal for efficient diagnostics and therapeutics.
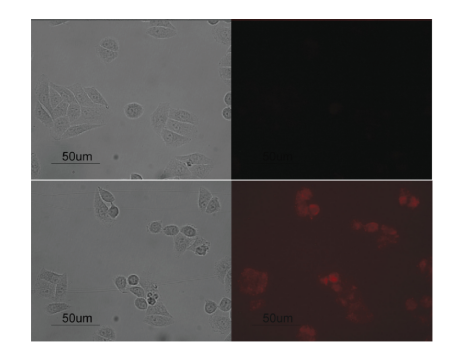
Figure 1. Fluorescence imaging pictures of HeLa cell lines treated with carboxyl-functionalized silica-coated QDs (upper row) and Tf-conjugated carboxyl-functionalized silica-coated QDs (bottom row). The panel on the left displays the transmission images of some cells, and the corresponding fluorescence images are shown in the right panel. (Qian, J., et al. (2010). Carboxyl-Functionalized and Bio-Conjugated Silica-Coated Quantum Dots as Targeting Probes for Cell Imaging. Journal of Nanoscience and Nanotechnology, 10(3), 1668–1675)
Although nanotechnology has grown fast and NPs may revolutionize therapy, imaging and early diagnosis of many diseases, there are some inherent problems need to be solved. Toxicity of NPs is the No. 1 problem. Due to having the tendency of coated or uncoated NPs accumulating in the liver, the detailed mechanism for exclusion from the body should be carefully studied. The other problems need to be addressed include minimizing the batch-to-batch variations when formulating NPs, warranting practical utility of the NPs by improving the synthetic yield and effective drug loading. Moreover, the development of safety guidelines by the government is in an urgent need, to control the environmental effects and the potential effects on human health when manufacturing the NPs.
CD Bioparticles is specialized in the development of drug delivery systems and customizing nanocarriers for drug delivery utilizing our core technologies. With our high-quality products and services, the efficacy of your drug delivery can be tremendously improved.
We offer custom synthesis of polymer microspheres and nanoparticles for medical diagnostics. Clients may select the material type, particle size, size distribution, color dye, fluorescent dye, and/or surface functional groups such as carboxyl or amine groups. We enable you to make wise decisions on all components of the nanoparticle structure so that the optimal candidate is identified for your target of interest in terms of stability, off-target toxicity, and efficacy. Besides, methodologies developed to produce the nanoparticles are simple, scalable and reproducible and ultimately suitable for large-scale manufacture.
References:
1. Parveen, S., Misra, R., & Sahoo, S. K. (2012). Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine: Nanotechnology, Biology and Medicine, 8(2), 147–166.
2. Medley, C. D., Smith, J. E., Tang, Z., Wu, Y., Bamrungsap, S., & Tan, W. (2008). Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells. Analytical Chemistry, 80(4), 1067–1072.
3. Corsi, F., De Palma, C., Colombo, M., Allevi, R., Nebuloni, M., Ronchi, S., Prosperi, D, et al. (2009). Towards ideal magnetofluorescent nanoparticles for bimodal detection of breast-cancer cells. Small, 5(22), 2555–2564.
4. Smith, J. E., Medley, C. D., Tang, Z., Shangguan, D., Lofton, C., & Tan, W. (2007). Aptamer-conjugated nanoparticles for the collection and detection of multiple cancer cells. Analytical Chemistry, 79(8), 3075–3082.
5. Valanne, A., Huopalahti, S., Soukka, T., Vainionpää, R., Lövgren, T., & Härmä, H. (2005). A sensitive adenovirus immunoassay as a model for using nanoparticle label technology in virus diagnostics. Journal of Clinical Virology, 33(3), 217–223.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.