As a new type of organic porous material, covalent organic frameworks (COFs) are mainly composed of small organic molecules connected to each other through strong covalent bonds. In addition to the excellent properties of other organic porous materials (such as microporous polymers (PIMs), conjugated microporous polymers (CMPs) and super crosslinked polymers (HCPs), etc.), including light weight, low density and specific surface area It can be used in fields such as gas adsorption and storage, industrial catalysis, drug delivery, etc. COFs also have the unique properties of regular crystal structure, open pores and uniform size, so they can be used as excellent catalyst carriers, organic electronic devices, and drugs sustained release carrier and molecular sieve film, etc. Compared with COFs, although metal organic frameworks (MOFs) have better crystallinity and have greater application value in gas storage and separation, supercapacitors, etc., when used in water, high temperature or strong acid-base environments, they will Because of the poor stability, the pore structure collapses, which reduces the actual use value of related composite materials. Since COFs have excellent chemical stability, it is necessary to continue to develop new COFs materials and systematically study them. There are many types of organic monomers for synthesizing COFs. In the process of synthesis, different types of monomers can be selected and the synthesis conditions can be controlled to design COFs of different types, topologies and pore sizes. Obviously, the structural flexibility of COFs makes it a material with great application potential. Compared with traditional porous materials, the research time of COFs is shorter, and its synthesis methods need to be explored and optimized, and the application fields need to be further expanded. Therefore, in order to deeply understand the synthesis process, structure control methods and application fields of COFs, this article has carried out a more comprehensive review of COFs materials.
COFs are a kind of attractive organic porous materials, mainly composed of specific organic monomers "stacked" in the form of reversible polycondensation reaction. Due to the differences in the types of these organic monomers, the reversible polycondensation reactions involved are also different, so that in the end, different organic monomers can be connected to each other with different types of covalent bonds to obtain various types of COFs. COFs can be divided into boric acids, triazines, imines, phenylhydrazones, keto-enamines, polyimides, Phthalocyanines and porphyrins. The reactions for synthesizing boric acid COFs include self-dehydration polycondensation of boric acid groups and dehydration polycondensation between boric acid groups and aromatic compounds with polyhydroxyl groups. Therefore, boric acid COFs are formed by the B-O covalent bond connection. Triazine COFs (CTFs) are formed by cyano trimerization of aromatic cyanide compounds and connected through triazine rings (-C3N3-). Due to the poor reversibility of the cyano trimerization reaction, the crystallinity of CTFs is also relatively poor. Imine COFs are formed by Schiff base polycondensation of aromatic aldehydes and aromatic amines connected by C = N covalent bonds. Phenylhydrazone COFs are formed by hydrazone bonds between hydrazide aromatic compounds and aromatic aldehydes. Keto-enamine COFs are the intermediate COFs (enol-imine COFs) that undergo Schiff base reaction between 1,3,5-trialdehyde phloroglucinol and some aromatic amines to synthesize the expected structure. Amine COFs undergo an irreversible proton tautomerism and are transformed into chemically more stable keto-enamine COFs. Polyimide COFs (PI-COFs) are formed by the imidization reaction of aromatic amines and aromatic dianhydrides, which are connected through imide rings (-CO-N-CO-). Phthalocyanine-based COFs and porphyrin-based COFs are two types of COFs with similar structures, which are respectively formed by phthalocyanine-based molecules and porphyrin-based molecules connected by B-O or C=N covalent bonds.
COFs are generally composed of light elements such as B, H, C, N and O, and have the advantages of light weight and low density. The orderly connection between the organic units ensures that COFs have the characteristics of uniform pores, high specific surface area and high crystallinity. The covalent bonds connecting COFs endow COFs with high temperature resistance, but the different covalent bonds of COFs make COFs different in other chemical stability. For boric acid-based COFs, although the empty p orbitals of boron in the BO covalent bond are conjugated with adjacent oxygen atoms and benzene ring, they are still vulnerable to moderate nucleophilic attacks, causing the hydrolysis of B-O covalent bond, which in turn leads to the collapse of its pores. In addition to boric acid COFs, COFs connected by other covalent bonds have good chemical stability in harsh environments such as general organic solvents, water, acids and alkalis. For example, Uribe-Romo et al. synthesized the imine COFs (COF-300) for the first time. COF-300 is insoluble in water and common organic solvents and can maintain permanent porosity. Based on the irreversibility of enol-keto-tautomerism, Kandambeth et al. successfully synthesized keto-enamine COFs (TpPa-1 and TpPa-2) with good chemical stability in water, strong acid and strong base environments.
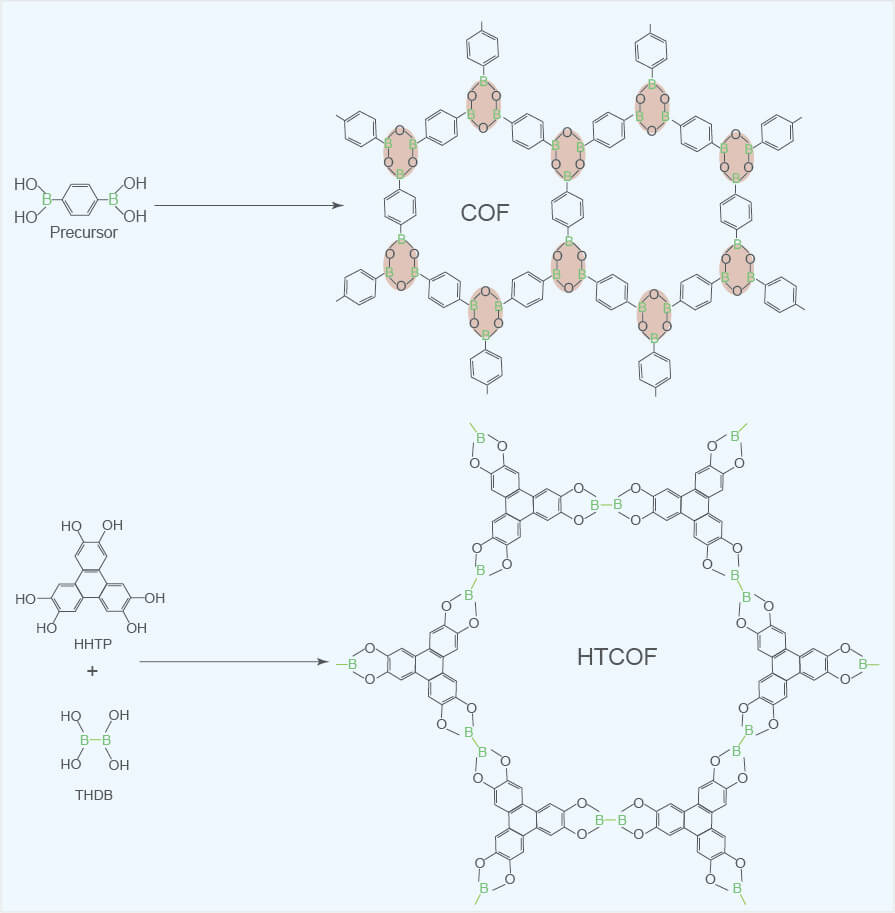
Figure 1. Schematic diagram of COFs formation.
Because of COFs' excellent characteristics, it has broad application prospects in the fields of gas adsorption, catalysis, drug delivery, organic electronic devices, and selective molecular sieve films. The current research progress of drug delivery has successfully demonstrated the great potential of COFs in the field of drug delivery. In 2015, Fang et al. applied COFs to the field of drug delivery for the first time, and designed and synthesized two 3D polyimide COFs (PI-COF-4, PI-COF-5). Among them, the two PI-COFs showed high loadings for ibuprofen (IBU), 24% (mass fraction) and 20% (mass fraction), respectively. After 12 h, the IBU releases of the two PI-COFs reached 60% and 49%. In 2016, Bai et al. loaded the anticancer drug 5-fluorouracil (5-FU) with synthetic PI-COF-3 and PI-COF-2 as carriers. The loading of 5-FU in 5-FU@ PI-COFs reaches 30% (mass fraction) and has good dispersibility in water. The survival rate of normal cells in 5-FU@ PI-COFs medium is as high as 80%, indicating that 5-FU@ PI-COFs has good biocompatibility. The survival rate of breast cancer cells (MCF-7 cells) placed in 5-FU@ PI-COFs-treated medium after 24 h is 40%, and after 48 h it is 10%, indicating 5-FU@ PI-COFs Has good drug release efficiency. Vyas et al. synthesized imine COFs (TTI-COF) with triazine structure, and successfully loaded quercetin on TTI-COF through the interaction between quercetin and the hydrogen bond in the TTI-COF imine bond. Studies have shown that TTI-COF loaded with quercetin can more effectively inhibit the growth of cancer cells, and TTI-COF does not produce any cytotoxicity to normal cells. In 2017, Mitra et al. performed three consecutive post-modifications on the synthesized TpASH, which weakened the π-π stacking effect between layers, and successfully peeled off TpASH into covalent organic nanosheets (TpASH-FA). TpASH-FA shows good targeted drug delivery function and has good dispersibility in water.
References:
1. Cote A P, et al.; Porous, Crystalline, Covalent Organic Frameworks. Science. 2005, 310( 5751): 1166-70.
2.
Kuhn P, et al.; Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew Chem Int Ed Engl. 2008, 47(18):3450-3.
3.
Feng X, et al.; Covalent organic frameworks. Chem Soc Rev. 2012, 41(18):6010-22.
4.
Uribe-Romo FJ, et al.; A crystalline imine-linked 3-D porous covalent organic framework. J Am Chem Soc. 2009, 131(13):4570-1.
5.
Uribe-Romo FJ, et al.; Crystalline covalent organic frameworks with hydrazone linkages. J Am Chem Soc. 2011, 133(30):11478-81.
6.
Fang Q, et al.; 3D Porous Crystalline Polyimide Covalent Organic Frameworks for Drug Delivery. J Am Chem Soc. 2015, 137(26):8352-5.
7.
Bai L, et al.; Nanoscale covalent organic frameworks as smart carriers for drug delivery. Chem Commun (Camb). 2016, 52(22):4128-31.
8.
Mitra S, et al.; Targeted Drug Delivery in Covalent Organic Nanosheets (CONs) via Sequential Postsynthetic Modification. J Am Chem Soc. 2017, 139(12):4513-4520.
9.
Kandambeth S, et al.; Construction of crystalline 2D covalent organic frameworks with remarkable chemical (acid/base) stability via a combined reversible and irreversible route. J Am Chem Soc. 2012, 134(48):19524-7.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.