Metal organic framework (MOFs) materials are a type of material with a repeating network structure formed by self-assembly of metal ions with organic ligands as the center. In recent decades, MOFs research has achieved breakthrough development. Due to the diversity of its crystal structure properties, adaptability and ultra-high surface area, MOFs have become a highly versatile platform in many fields such as gas adsorption and separation, chemical sensing, proton conductivity, and biomedicine, with potential applications prospect. MOFs use evaporative solvent method, diffusion method (also subdivided into gas phase diffusion, liquid phase diffusion, gel diffusion, etc.), hydrothermal or solvothermal methods, ultrasonic and Synthesized by microwave method and other synthetic methods. Among these synthesis methods, hydrothermal or solvothermal methods are the most important, and most MOFs are synthesized by hydrothermal or solvothermal methods.
The hydrothermal or solvothermal method belongs to the category of liquid-phase chemical method, which refers to a chemical synthesis method carried out under high temperature and high pressure in a sealed pressure vessel with water as a solvent. Researchers can synthesize MOFs with different morphologies, sizes, specific functions, and specific pore size distributions by selecting appropriate organic ligands and metal elements, controlling synthesis conditions, and selecting appropriate nucleation and growth methods. Compared with traditional inorganic porous materials, MOFs have a larger specific surface area and porosity. It is especially important that the organic ligands of MOFs can be further modified to achieve purposeful functionalization.
The most important feature of MOFs material is its variable metal center and organic ligands, which leads to the diversity of its structure and function. The choice of metal centers covers almost all metals, among which Zr, Fe and Cu are more widely used. The difference in metal valence and coordination ability also led to the emergence of different types of MOFs materials. Moreover, a wide variety of ligands such as carboxylic acids and imidazole derivatives are available for selection and modification, as well as the preparation of MOFs materials hybridized with a variety of organic ligands, which broaden the application range of MOFs materials. Due to the presence of small solvent molecules such as dimethylformamide (DMF), ethanol, and water in the synthesized MOFs, the unsaturated metal center combines with these small solvent molecules to meet the coordination requirements. These small solvents are removed by heating or vacuum treatment. Molecules can then reappear the unsaturated metal binding sites of MOFs. These unsaturated metal binding sites can coordinate with substances with amino or carboxyl ion groups, so that MOFs materials can be used in biomedical fields such as drug carriers, gene carriers, or peptide separation.
MOFs are currently a popular drug delivery method. Clinical studies have shown that by selecting a suitable drug carrier, the release, absorption, metabolism and excretion process of the drug in the human body can be optimized, thereby increasing the utilization rate of the drug and reducing the toxic side effects of the drug. MOFs have high specific surface area and large pore size that can encapsulate drugs, and the internal structural characteristics of MOFs can be changed by adjusting the size and properties of the encapsulated substance. The instability of the coordination bond between the metal and the organic ligand leads to its biodegradability. Therefore, MOFs have become a research hotspot for targeted drug delivery carrier materials. Modification of them can not only achieve high drug loading, but also The appropriate release rate can be controlled. In addition, MOFs as a drug controlled release carrier have a simple synthesis method, good stability and strong targeting, and have become one of the new type of drug controlled release carrier materials with excellent performance. Interestingly, MOFs materials are used in biological applications to form low-toxic nanocarriers by selecting core metal centers. At present, Fe, Zn, Zr, Mn, Mg and Cu are widely used in the construction of MOFs because of their low toxicity measured by oral lethal dose (LD 50).
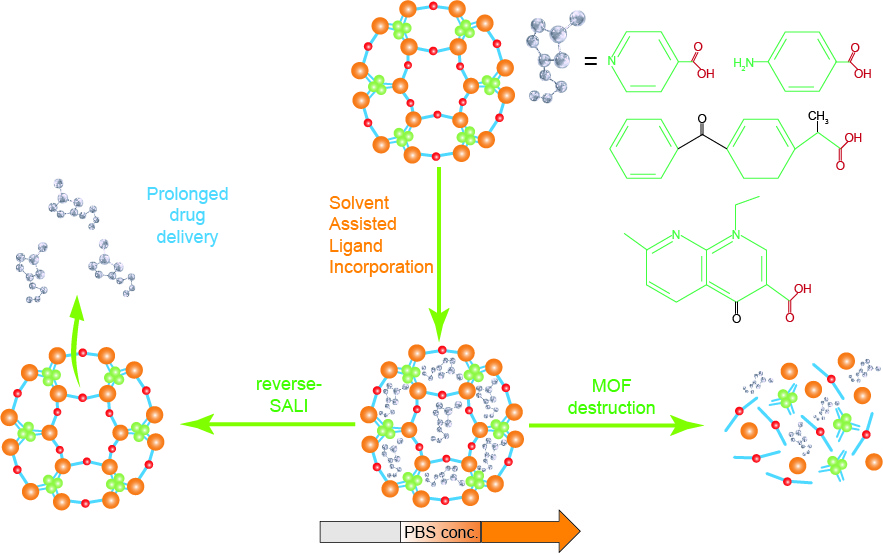
Figure 1. Mesoporous MOF as drug delivery system.
According to statistics, since the first report of drug loading based on MOFs in 2006, many researchers have been The drug delivery system of MOFs has been intensively studied. Regarding drug loading and release, taking doxorubicin hydrochloride nanoparticles as an example, the drug and the material are not simply adsorbed, but coordinated with the remaining metal ion sites, and the intensity of the coordination is affected by pH. Therefore, the release rate and maximum cumulative release rate of doxorubicin hydrochloride are affected by pH, so it has a certain sustained release ability. MOFs nanocarriers can increase the persistence and stability of the drug in the cell, release it at the site most conducive to the treatment of the disease, and can reduce the amount of drug used for the disease to reduce or avoid the toxic side effects caused by the drug.
References:
1. DUAN F, et al.; A simple and powerful co- delivery system based on pH-responsive metal-organic frameworks for enhanced cancer immunotherapy.Biomaterials. 2017, 122: 23-33.
2. BAEZA A, et al.; Recent advances in porous nanoparticles for drug delivery in antitumoral applications: inorganic nanoparticles and nanoscale metal-organic frameworks. Expert Opin drug Deliv. 2017, 14(6): 783-796.
3. Lee Y -R, et al.; Synthesis of Metal-Organic Frameworks: A Mini Review. Korean J. Chem. Eng.. 2013, 30: 1667-1680.
4. Vleet M J V, et al.; In Situ, Time-Resolved, and Mechanistic Studies of Metal–Organic Framework Nucleation and Growth. Chemical Reviews. 2018, 118(7).
5. Sun CY, et al.; Chiral nanoporous metal-organic frameworks with high porosity as materials for drug delivery. Advanced materials. 2011, 23(47): 5629-5632.
6. Schneemann A, et al.; Flexible metal-organic frameworks. Chemical Society Reviews. 2014, 43(16): 6062-6096.
7. Orellanatavra C, et al.; Amorphous metal-organic frameworks for drug delivery. Chemical Communications. 2015, 51(73): 13878-13881.
8. Gu Z Y, et al.; Metal-organic frameworks for efficient enrichment of peptides with simultaneous exclusion of proteins from complex biological samples. Chemical Communications. 2011, 47(16): 4787-4789.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.