Drug development for lung-specific conditions requires advanced expertise and technologies. Our team offers cutting-edge solutions including nanocarriers, viral vectors, ligand modifications, activity evaluation, and more. CD Bioparticles' goal is to accelerate the development of effective treatments for lung diseases and conditions. CD Bioparticles' nanotechnology-based solutions provide precise drug delivery to the site of action in the lung. Nanocarriers can be designed with specific physiochemical properties to improve drug efficacy, stability, and bioavailability. We provide tailored services in drug delivery system design and synthesis, characterization, and optimization.
The lungs are directly accessible from the airway, drug delivery into the respiratory bronchiole and air sacs of the lung is commonly through inhalation. The inhalation route offers several advantages, including non-invasive site-specific delivery, a large alveolar surface area for absorption, and lower first-pass clearance compared with the oral route[1]. Inhalation of medical drug particles through human airway is widely utilized for the remedy of lung diseases, such as respiratory infection, asthma, chronic obstructive pulmonary disease, lung cancer and so forth[2]. There are four types of cells that are present in the alveolar region of the lungs, including the alveolar macrophages, epithelial type I and II cells and the alveolar brush cells (type III). Drug administration through inhalation often requires multiple daily administrations, largely because of rapid clearance of drugs from pulmonary tissue. Particles <1 µm are capable of avoiding mucociliary clearance by penetrating mucus network into epithelial cells. On the other hand, Accepted Manuscript particles with sizes of 1-5 µm often deposit in alveolar regions, while particles > 5 µm deposit in the upper airways. The types of nano delivery systems used for the treatment of lung diseases are shown in Figure 1.
Lung-targeted drugs administered through intravenous injection can achieve high targeting specificity through surface modification with specific antibodies and peptides. In contrast, inhalation therapy is a relatively emerging approach.
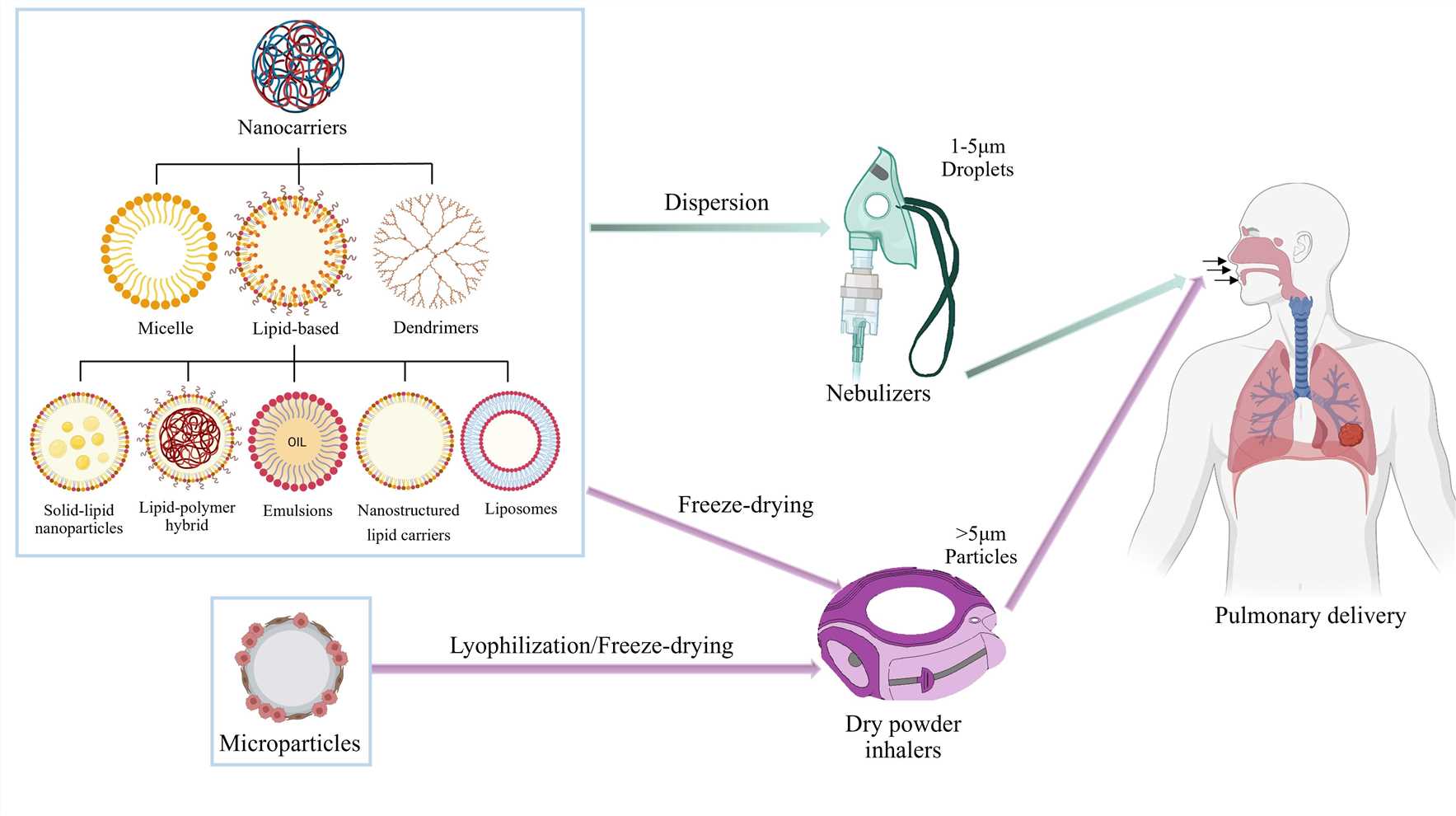
Figure 1 Types of drug delivery systems targeting the lungs[3].
Targeted drugs for the lungs, in addition to various monoclonal antibodies for the treatment of lung cancer, also come in various forms and are being developed and researched through multiple routes of administration. Table 1 lists some FDA-approved nanoparticle-based lung-targeted drugs for your reference.
Table 1 FDA-approved Lung-targeting drugs
| Name | Ingredient Active | Carrier | Indication | Date of Approval |
|---|---|---|---|---|
| Curosurf® | Proteins SP-B and SP-C | Liposomes | Lung activator for stress disorder; pulmonary surfactant for respiratory distress syndrome | FDA (1999) |
| Abraxane® | Paclitaxel (ABI-007) | Albumin-bound paclitaxel nanoparticles | Breast cancer; non-small cell lung cancer and pancreatic cancer | FDA (2005; 2012; 2013) |
| Rapamune® | sirolimus (Rapamycin) | Nanocrystal |
A rare progressive lung disease (Lymphangioleiomyomatosis) |
FDA (2015) |
| Lipusu® | Paclitaxel | Lipid-based Nanoparticles | breast cancer, non-small-cell lung cancer (NSCLC) | FDA (2016) |
References:
1. Zhu Y, Xu J, Lian S, Zhang R, Hou J, Wang M, Yan X: Difference Analysis Between Canine Adenovirus Types 1 And 2. Front Cell Infect Microbiol 2022, 12:854876.
2. Wu C, Yan W, Chen R, Liu Y, Li G: Numerical study on targeted delivery of magnetic drug particles in realistic human lung. Powder Technology 2022, 397.
3. Gupta C, Jaipuria A, Gupta N: Inhalable Formulations to Treat Non-Small Cell Lung Cancer (NSCLC): Recent Therapies and Developments. Pharmaceutics 2022, 15(1).
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.