CD Bioparticles has abundant experience in designing and producing targeted drugs, with a focus on nanoparticle and virus-based liver targeting drug delivery systems. We can design and produce surface-modified, highly pure, efficiently targeted, and low cytotoxicity nanoparticles for you based on the cell type and characteristics you want to target, or provide pseudovirus products and services characterized by high virus effectiveness, expression efficiency, and low immunogenicity. CD Bioparticles' platform has rich production experience and can provide strong support for drug targeting research. CD Bioparticles can also assist in evaluating the in vitro and in vivo liver targeting drug activity, and is committed to helping clients achieve high drug concentrations in the liver while minimizing systemic exposure.
The liver is a vital organ that plays a crucial role in maintaining our overall health by processing and removing toxins from our bodies. However, liver disease is a growing concern worldwide, with conditions such as non-alcoholic fatty liver disease (NAFLD), hepatitis, and liver cancer on the rise. In fact, liver disease is now the fourth leading cause of death in the United States.
Because the liver is responsible for metabolizing drugs and removing them from the body, untreated liver diseases can greatly affect the effectiveness and safety of drugs. This is why there is an urgent need for liver-targeted drugs. These types of drugs are specifically designed to reach the liver and deliver their therapeutic effects while minimizing their impact on other organs.
Liver-targeted drugs can also increase the bioavailability and reduce the toxicity of drugs, making them more effective and safer for patients. They can also improve treatment outcomes for liver diseases, such as NAFLD and hepatitis, by targeting specific mechanisms that are involved in the pathogenesis of these diseases.
Unlike most other tissues, the liver does not exhibit impermeable basal lamina. In the absence of interfering mechanisms such as aggregation or protein binding, the majority of nanomedicines exhibit rapid passive liver accumulation following systemic administration. In the meanwhile, cells in the liver express a variety of receptors that are potentially useful for drug active targeting.
Liver-targeted drug delivery systems are based on passive or active strategies[1]. Passive strategies rely on the accumulation of nanomedicines in the liver to achieve an increased local drug concentration and at the same time minimization of non-specific transport to other organs and consequently reduce adverse side effects. This is typically achieved by tuning physical properties such as the size, surface modifications to reduce non-specific binding, or by locoregional application (Figure 1). Accordingly, the passive accumulation of nanomedicines at a specific body site relies on certain anatomical and pathophysiological features complementing the nanocarriers' properties.
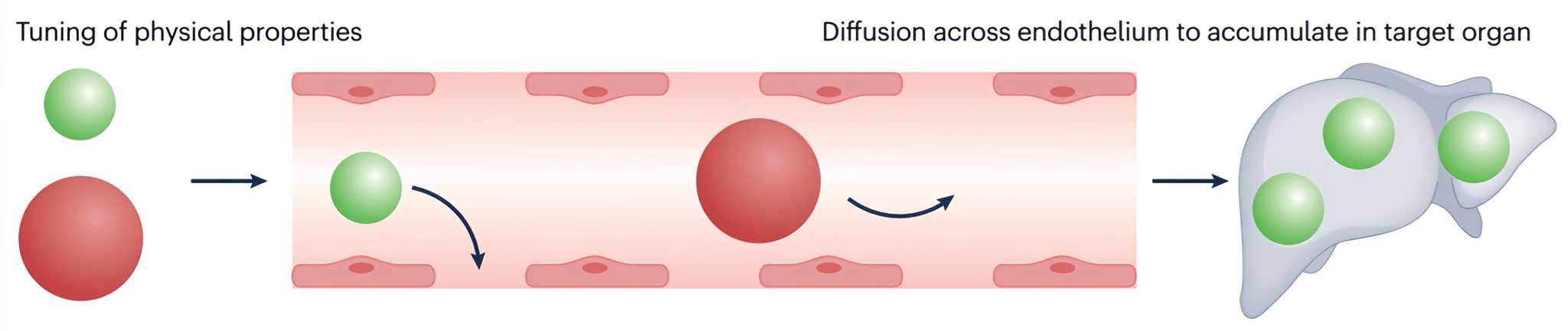
Figure 1 Mechanisms of passive targeting[2]
Active targeting utilizes surface conjugation with a cell-specific ligand such as a peptide, protein, antibody, or carbohydrate, which binds to the corresponding receptor at the cell membrane. This approach has the advantage of being more directed to a specific liver cell type that is relevant to a disease while reducing the side effects on other liver cell types. Please refer to Figure 2 for the targeted mechanism.
Viral vectors such as various subtypes of adeno-associated virus have obvious hepatic tropism and can be used as drug delivery vehicles when targeting nucleic acid drugs targeting the liver.
Modern targeting technologies often combine both strategies to first facilitate general (passive) liver uptake and subsequently ligand mediated (active) cellular internalization for efficient and precise treatment.
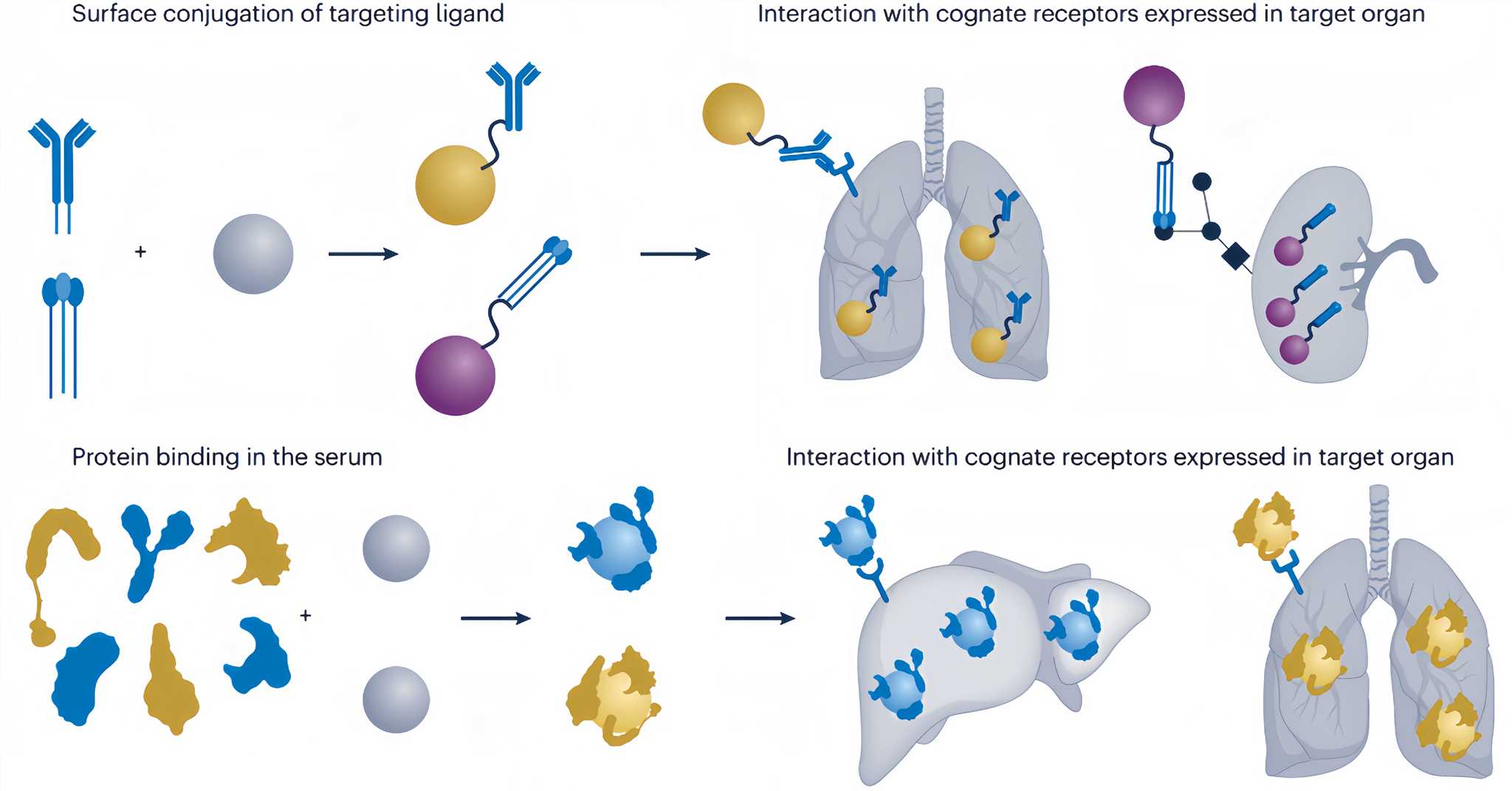
Figure 2 Mechanisms of active targeting[2]
Most advanced materials such as nanocarriers or viral vectors have been used for liver-targeted drug delivery systems. In the 1990s Abelcet and AmBisome (liposomal amphotericin B) were approved for treating advanced fungal infections that often reside in the macrophages, including the KCs. Negatively charged mediated opsonization that favors recognition by the KCs following i.v. administration. In 2018, ONPATTRO was approved for TTR amyloidosis, and the delivery mechanism is through specific binding of serum apolipoproteins for active targeting to the hepatocytes. In 2020, CSPC ZhongQi Pharmaceutical Technology developed PLM60 (PEGylated liposomal mitoxantrone) for HCC[3].
Table 1 List of receptors on specific liver cell types for targeted delivery.
| Hepatocyte | Hepatic stellate cells | Kupffer cells | Sinusoidal endothelial cells | ||||
|---|---|---|---|---|---|---|---|
| Transferrin receptors | Transferrin | Collagen type VI Receptor |
cRGD* peptide C*GRGDSPC* peptide |
Mannose receptor |
Mannose 4-aminophenyl-α-d-mannopyranoside |
Low-density lipoprotein receptor | KLGR peptide |
| Heparan sulfate proteoglycans | CKNEKKNKIERNNKLKQPP peptide | Mannose 6-phosphate receptor | Mannose 6-phosphate | Fucose receptor |
Fucose 4-aminophenyl-β-l-fucopyranoside |
Scavenger receptors | Aconitylated human serum albumin |
| Scavenger receptor B1 | Apo A-I | Platelet-derived growth factor receptor-β | Cyclic C*SRNLIDC* peptide | Galactose receptor | Galactose | Hyaluronan receptor | Hyarluronic acid stearylamine |
| Low-density lipoprotein receptor | Apo E | Retinol binding protein | Retinol | Scavenger Receptors | IgG and calcitriol | ||
| Folate receptor | Folate | Fc Receptors | CC52 antibody | ||||
| C-X-C chemokine receptor type 4 | Plerixafor | ||||||
| Asialoglycoprotein receptor | Galactose; N-acetyl galactosamine; Asialofetuin; PreS1 peptide; Lactose; Lactosyl-norcantharidin; Soybean sterylglucoside; Pullulan | ||||||
References:
1. Zhou F, Teng F, Deng P, Meng N, Song Z, Feng R: Recent Progress of Nano-drug Delivery System for Liver Cancer Treatment. Anticancer Agents Med Chem 2018, 17(14):1884-1897.
2. Dilliard SA, Siegwart DJ: Passive, active and endogenous organ-targeted lipid and polymer nanoparticles for delivery of genetic drugs. Nature Reviews Materials 2023.
3. Bottger R, Pauli G, Chao PH, Al Fayez N, Hohenwarter L, Li SD: Lipid-based nanoparticle technologies for liver targeting. Adv Drug Deliv Rev 2020, 154-155:79-101.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.