Freeze-dried Flash Release Tablets Based Drug Delivery System
CD Bioparticles is a supplier specializing in the research of freeze-dried flash-release tablets. CD Bioparticles has an experienced and professional R&D team and state-of-the-art laboratory equipment to develop freeze-dried flash-release tablets with different active substances. We offer a bespoke design service to produce the perfect packaging.
Introduction of Freeze-dried Flash Release Tablets
Freeze-dried Flash Release Tablets is a novel formulation of active biological agents, which are converted into dry powders or tablets by freeze-dry technology in order to prolong their shelf-life improve stability, and improve the solubility and releasing profile. It is a tablet that disintegrates or dissolves rapidly in the mouth without water. The active ingredients can be absorbed through the mucous membranes of the mouth or esophagus, and can also enter the stomach and intestines with the help of swallowing forces, where they are absorbed through the digestive system and take effect. Frozen flash release technology is a landmark revolutionary dosage form innovation in the world and has been widely used in the fields of food, healthcare products, pharmaceuticals and cosmetics. In the field of pharmaceuticals, it can be developed into dosage forms such as orally disintegrating tablets (lyophilized) and sublingual tablets (lyophilized). In the field of food products, it can be developed into the form of containing sugar, solid condiments and so on.
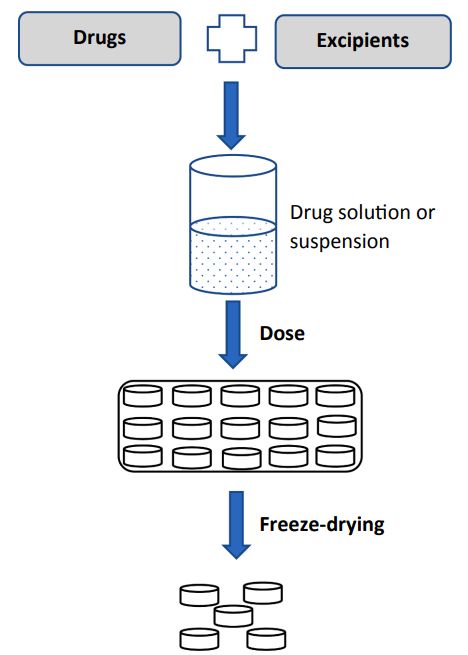 Figure 1. Illustration of basic steps to produce freeze-dried tablets from a solution or a suspension.[1]
Figure 1. Illustration of basic steps to produce freeze-dried tablets from a solution or a suspension.[1]
Advantages of Freeze-dried Flash Release Tablets
- Rapid disintegration, flash release
Flash release effect allows active ingredients to be released and dispersed quickly
- Transmembrane absorption
Proprietary polymer excipients allow small molecules of active ingredients to be widely coated in the oral cavity and digestive tract mucosa, creating favorable conditions for transmembrane absorption
- Fast-acting
The active ingredients are absorbed in the oral mucosa, and can directly participate in the systemic circulation to shorten the maximum blood concentration (Cmax) peak time (Tmax)
- Safe to take, reduce the dose
Oral mucosal absorption can avoid and reduce the hepatic and intestinal first-pass effect, thus greatly reducing the same effect of the application of the dose of ingredients, improve safety. Improve the bioavailability and reduce the dose
- Low adverse effects
The drug is absorbed through the oral mucosa, not through the gastrointestinal tract, which can reduce the occurrence of gastrointestinal adverse reactions to drugs
- Low amount of excipients, high safety
No swallowing pressure, which can reduce the risk of choking, accidental inhalation and suffocation in children; small amount of excipients, which can reduce the risk of excipient allergy
- High stability, easy to use
Remove 95% ~ 99% of water by freezing, suitable for the preservation of active ingredients. It can be taken without water
The Applications of Freeze-dried Flash Release Tablets
- Medicine
1) For people with active medication difficulties (children and people with mental illness) and passive medication difficulties (the elderly and people with swallowing difficulties)
2)Development of fast-acting drug formulations (e.g. pain, insomnia, nausea and allergies)
3) People who have difficulty in drinking water. People who are bedridden, work in the field, and travelers who have difficulty in urination
- Food and health food
- Pet health
- Cosmetics
Our Featured Services
CD Bioparticles has a full range of leading experts in drug delivery. We can offer you a wide range of services related to drug delivery systems based on Freeze-dried Flash Release Tablets:
- Formulation Design: Based on the physicochemical properties of the drug and the dosage to be administered, we offer one-on-one design of freeze-dried flash release tablets by selecting the appropriate type of excipients.
- Performance Evaluation: We test the disintegration rate, stability of the active substance and maximum blood concentration of the tablets.
Quotations and Ordering

Reference
- Phuong HL, et al.; Strategies and formulations of freeze-dried tablets for controlled drug delivery. International Journal of Pharmaceutics. 2021, 597: 120373-120380.
 Figure 1. Illustration of basic steps to produce freeze-dried tablets from a solution or a suspension.[1]
Figure 1. Illustration of basic steps to produce freeze-dried tablets from a solution or a suspension.[1]