HSV Vectors
CD Bioparticles offers a reliable and efficient packaging system using amplicon vector for Herpes Simplex Virus vectors, ideal for both gene therapy and scientific research, especially neurological therapy. Using advanced techniques and reagents, we ensure the high titer, purity, viability, and consistency of our HSV vector services. Additionally, we offer a range of toxicological experiments and biodistribution research services to meet your specific research needs.
Introduction to HSV Vectors
Herpes simplex virus, the major human pathogen whose lifestyle is based on a long-term dual interaction with the infected host characterized by the existence of lytic and latent infections, has allowed the development of potential vectors for several applications in human healthcare1.
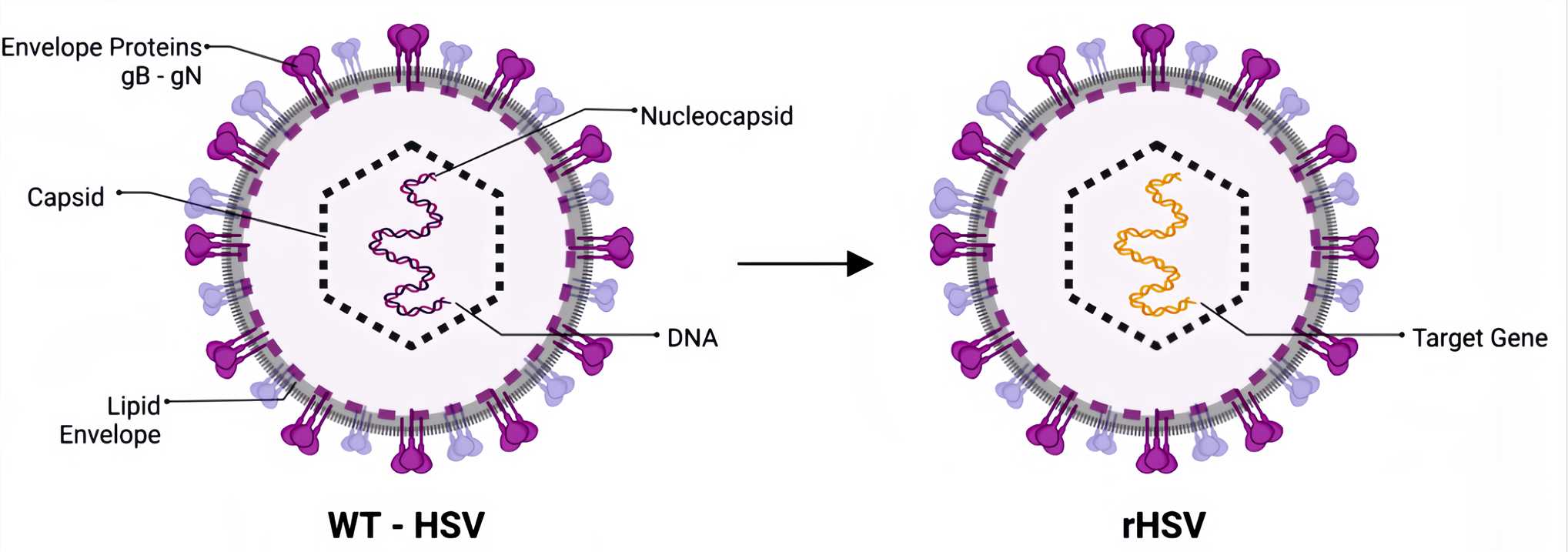 Figure 1. WT-HSV and Recombined HSV.
Figure 1. WT-HSV and Recombined HSV.
The Specific Features of HSV Vectors Include:
-
Large packaging capacity: HSV vectors can accommodate up to 50kb of foreign DNA;
-
High transduction efficiency: HSV vectors can transduce both dividing and non-dividing cells with high efficiency;
-
Long-term gene expression: HSV vectors can provide stable gene expression for long periods;
-
Neuronal tropism: HSV vectors are highly neurotropic, making them ideal for gene therapy applications for neurological disorders;
-
Low immunogenicity: Because HSV vectors are derived from a virus that is naturally present in human neuronal cells, they have low immunogenicity.
-
Versatility: HSV vectors can be modified to express various therapeutic genes and can be used in combination with other vectors to achieve desired therapeutic outcomes.
-
Non-integrating.
The Applications of HSV Vectors Include:
-
Gene therapy: HSV vectors are often used in gene therapy to deliver genes to specific cells in the body. This can help treat genetic disorders and other medical conditions.
-
Neural research: HSV vectors are also commonly used in neuroscience research to study the brain and nervous system.
-
Cancer treatment: HSV vectors can be used to deliver anti-cancer agents to tumor cells or as oncolytic viral vector, improving the efficacy of cancer treatment and become an effective activator of innate and adaptive immunity, such as G47∆ and T-VEC2.
-
Vaccine development: HSV vectors have also been explored as a way to develop vaccines for various diseases such as HSV-1 infection or other infectious diseases3.
-
Drug development: HSV vectors are being investigated as a tool for drug development, particularly in the realm of gene editing and protein production.
Our HSV Vectors Featured Services:
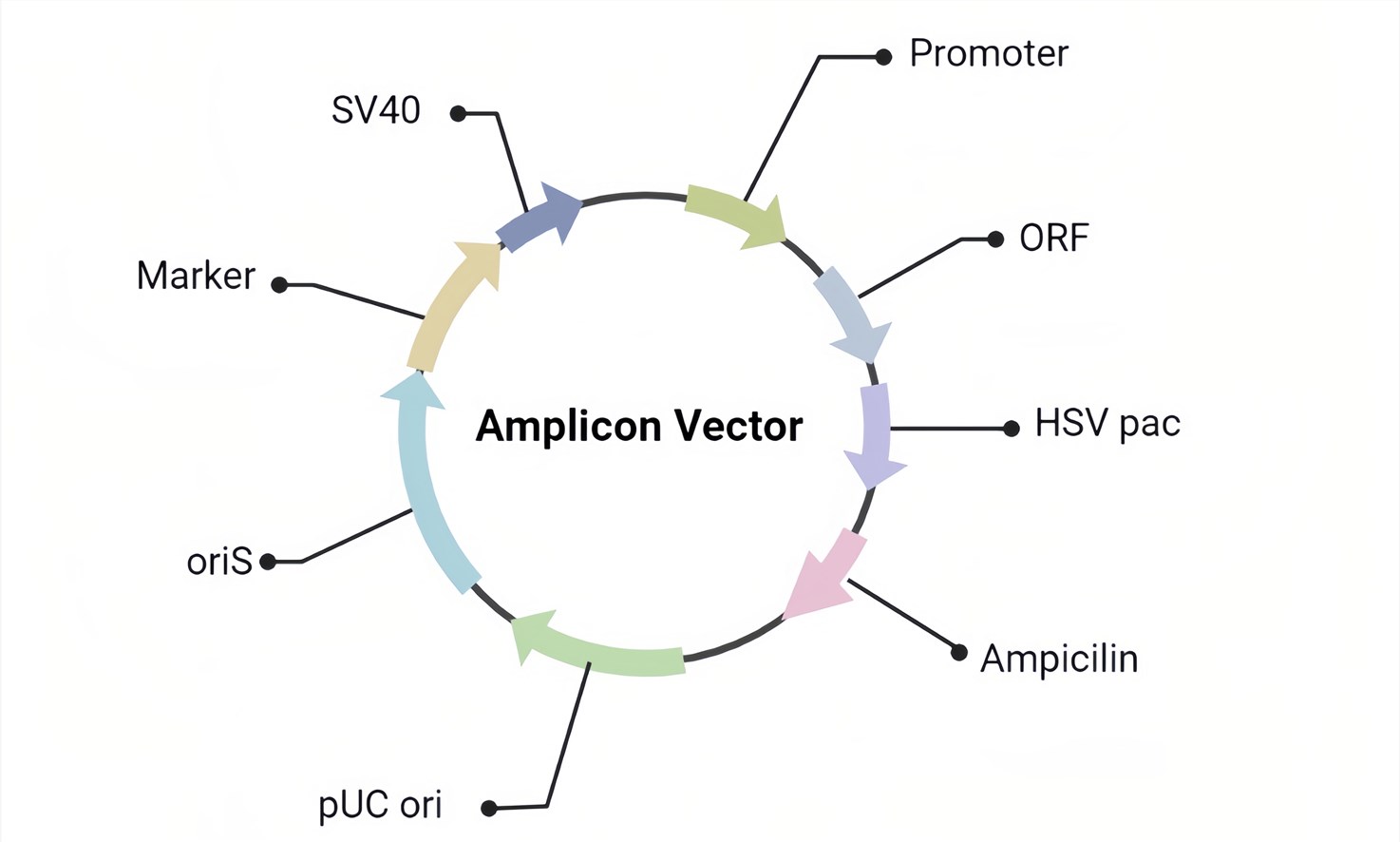 Figure 2. The basic design of the HSV amplicon vector.
Figure 2. The basic design of the HSV amplicon vector.
-
HSV packaging service
-
A. The Amplicon Vector (Figure 2) we used contains only the ori and pac of HSV genomics which can provide the maximum capacity of cargo.
-
B. The packaged pseudovirus products can contain one or more target genes, wrapped in the envelope of HSV virus.
-
C. The pseudovirus is replication-deficient but infectious.
-
D. EGFP or LacZ can be contained to determine transduction.
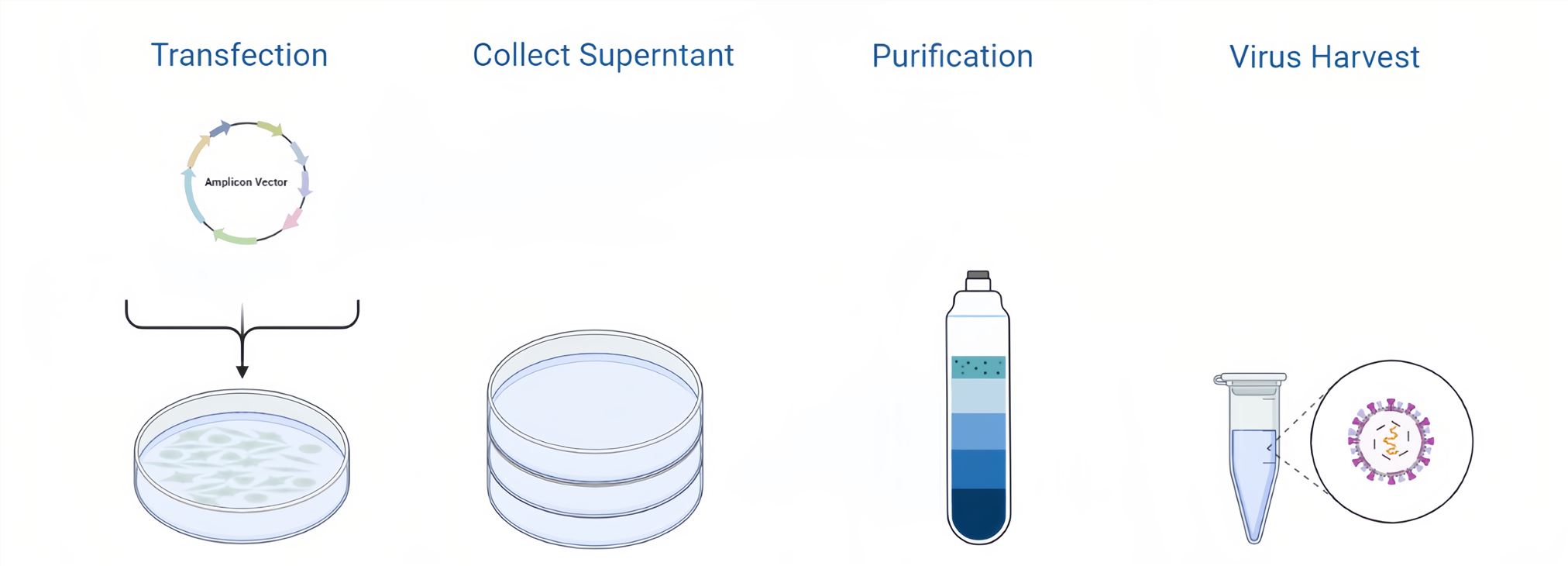 Figure 3. HSV Packaging Process.
Figure 3. HSV Packaging Process.
-
HSV quality control: Titer testing, sterility testing or mycoplasma testing according to application requirements.
-
Testing in vitro and in vivo: localization studies, infection rate, gene expression assessment, immunogenicity assessment, in vivo distribution, in vivo metabolism, off-target evaluation, toxicological evaluation, etc.
We are committed to providing you with excellent services and products. Please contact us if you have questions.
Quotations and Ordering
We would be glad to discuss your project in more detail with our support team if you would like more information.

References
-
Marconi P, Argnani R; et al. HSV as a Vector in Vaccine Development and Gene Therapy. In: Madame Curie Bioscience Database [Internet]. Austin (TX): Landes Bioscience; 2000-2013.
-
Su, Y., Su, C.; et al. Current landscape and perspective of oncolytic viruses and their combination therapies. Transl Oncol 25, 101530 (2022).
-
Stanberry LR. Clinical trials of prophylactic and therapeutic herpes simplex virus vaccines. Herpes. 2004;11 (Suppl 3):161A–169A.
 Figure 1. WT-HSV and Recombined HSV.
Figure 1. WT-HSV and Recombined HSV.
 Figure 2. The basic design of the HSV amplicon vector.
Figure 2. The basic design of the HSV amplicon vector.
 Figure 3. HSV Packaging Process.
Figure 3. HSV Packaging Process.
