CD Bioparticles uses 2nd or 3rd generation lentiviral vector packaging systems to provide customers with high-quality lentiviral vectors, ideal for gene therapy, construction of stable cell lines and scientific research applications. We have perfected a range of techniques and reagents that have significantly improved the titer, purity, and consistency of lentiviral vectors, ensuring reliable and consistent results. Moreover, we can provide you with various toxicological and biodistribution experimental research services to meet your specific scientific research requirements.
Lentivirus belong to the retroviridae family. The gene integrating the lentiviral vectors can be expressed in long-term. The lentiviruses are able to express multiple genes on high-level in one location and have robust packaging capacity of tolerant up to 9 kb external gene. Lentivirus vector platform can achieve target tissue specifically by employing tissue-specific promoters to regulate transgene expression. In addition, applying endogenous miRNAs for post-transcriptionally regulation can reduce immune response against the foreign gene(s). Researcher and new drug developers combined CRISPR technique and next-generation vaccine design with lentivirus delivery system to large-scale screen the drugs and vaccine candidates.
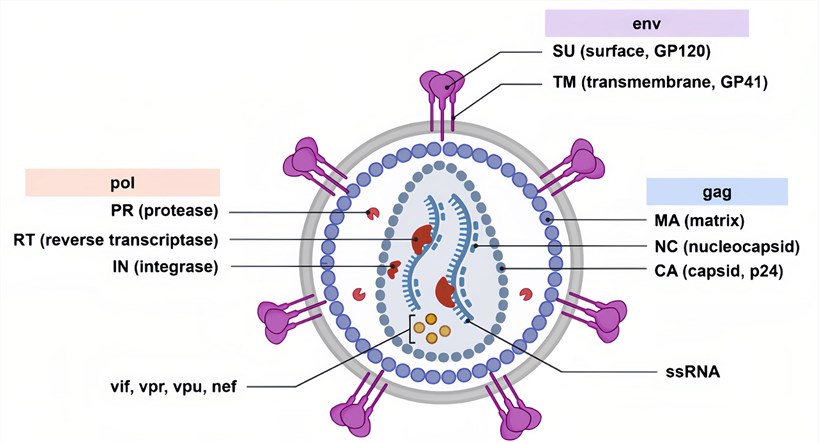 Figure 1. A schematic overview of the lentivirus HIV-1.
Figure 1. A schematic overview of the lentivirus HIV-1.
Gene therapy strategies based on lentivirus platform in vitro have had substantial advancements of treating genetic diseases, such as treatment of β-thalassemia, cerebral adrenoleukodystrophy, and treatment of inherited primary immune deficiency disorders.
CD Bioparticles has developed a series of proprietary technologies and reagents to improve the level of lentivirus packaging technology in terms of virus purity, titer, activity and consistency. Non-purified lentivirus can be used to transduce cells cultured in vitro (research-grade). The purified lentivirus can be used not only for cell transduction in vitro, but also for cell transduction in animals.
CD Bioparticles pseudoviruses have the advantages of low cytotoxicity (ultracentrifugation purification process), no mycoplasma pollution, no endotoxin, no exogenous microorganisms, and no host nucleic acid residues.
We are committed to providing you with excellent services and products. Please contact us if you have questions.
Quotations and Ordering

Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.