CD Bioparticles provides custom services for Cationic Nanoemulsions (CNEs) formulation design and drug encapsulation. Together with advanced preparation technologies for various liposomes, scientists in CD Bioparticles are proficient in producing CNEs for various types of drug loading, especially for poorly soluble drugs, nucleic acid and protein drugs, etc. We can provide the service of formulation design depending on the application, drug encapsulation, physical and chemical properties, in vitro and in vivo characterization according to customer's project needs.
CNEs are colloidal dispersions of two immiscible liquids, oil and water, in which one is dispersed in the other with the aid of a surfactant/co-surfactant mixture, the oil phase comprises cationic lipids, such as DOTAP and DOTMA, and/or PEG-lipids[1].
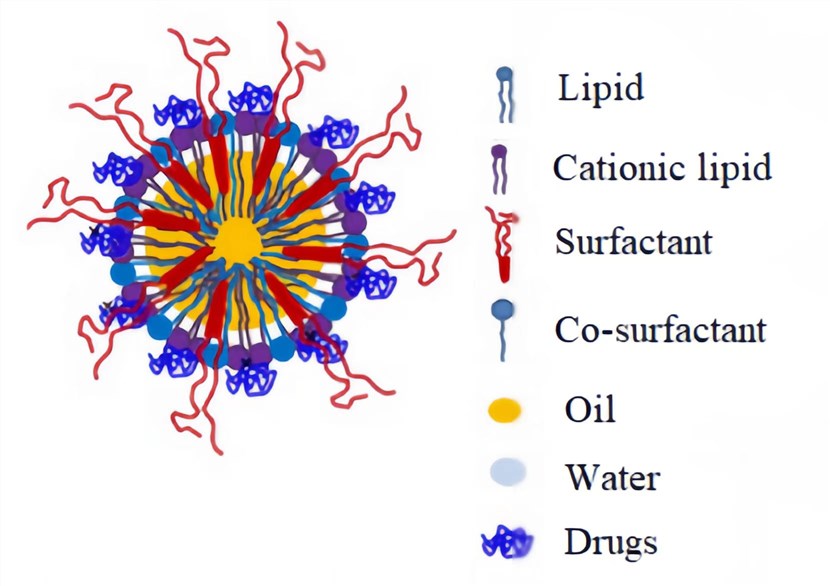 Figure 1. Schematic representation of RNA Cationic Nanoemulsions[1]
Figure 1. Schematic representation of RNA Cationic Nanoemulsions[1]
CNEs can promote the improvement of the bioavailability of drugs mainly through electrostatic interaction and mucosal adsorption so that they show broad application prospects in oral, mucosal, ocular and transdermal drug delivery systems.
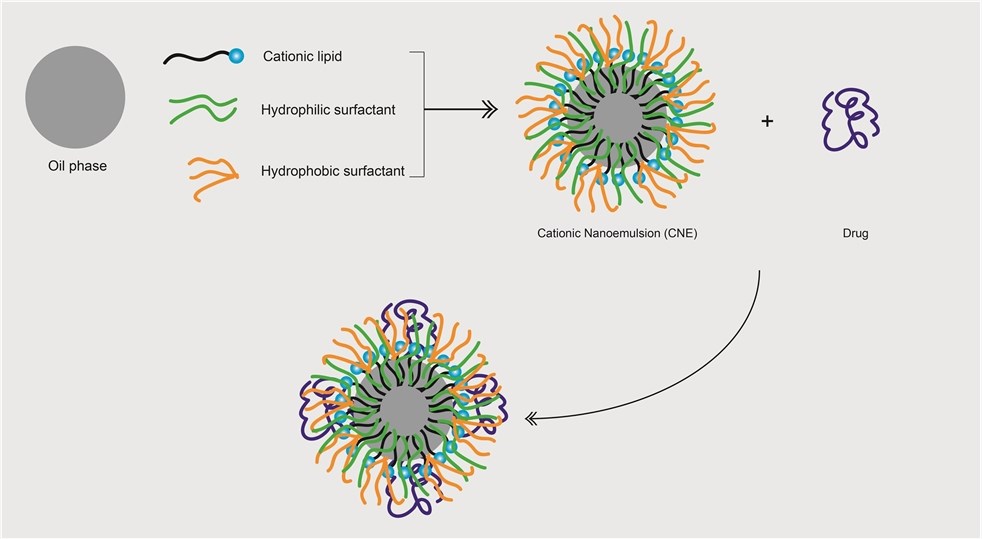 Figure 2. Cationic nanoemulsions drug loading process.
Figure 2. Cationic nanoemulsions drug loading process.
CNEs formulation design: we customize CNEs design based on our clients' demand by varying lipid structure (both commercially avaiable or early developed), lipid compositions, vesicle sizes, surface charges, etc.
CNEs encapsulation: we employ customized protocols to encapsulate drug molecules into LNPs by several different methods with high encapsulation efficiency.
CNEs-drug complex analysis: we can offer comprehensive analysis assays for CNEs before and after encapsulation, which includes visual appearance, size distribution, stability, zeta potential, lamellarity, entrapment efficiency, and release rate.
CNEs-drug biological activity characterization: we can control and analyze the cell uptake efficiency and the cytotoxicity by the lipid structure, charge ratio,etc.
Quotations and Ordering

Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.