CD Bioparticles provides custom services for conventional liposomes formulation design and drug encapsulation. Together with advanced preparation technologies for various liposomes, Scientists in CD Bioparticles are proficient in producing liposomes for various types of drug loading.
Conventional liposomes, the first generation of liposomes, are a family of vesicular structures based on lipid bilayers surrounding aqueous compartments (figure 1). These particles are typically composed of only phospholipids (neutral and/or negatively charged) and/or cholesterol without modification. With its hydrophobic lipid bilayer and the hydrophilic aqueous space, various types of drug compounds could be incorporated accordingly. Off-the-shelf particles are normally supplied as lyophilized or pre-liposome powder form. Custom made liposomal formulation can be rationally designed with high versatility to increase drug delivery efficiency, by being very flexible systems through changing their physicochemical properties such as size, lipid composition, surface charge and number and fluidity of the phospholipid bilayer. Compared to free drugs, conventional liposomal formulations enhance drug delivery by reducing the toxicity of compounds, improving drug biodistribution, modifying pharmacokinetics.
Most early work on liposomes as a drug-carrier system employed this type of liposomes to extend its clinical potential. Conventional liposome is the most common drug carrier by involving in the majority of marketed liposomal-based therapeutics and products in clinical development. However, they tend to fuse and/or aggregate with each other resulting in immature release of liposomal payload over time. In addition, conventional liposomes underwent rapid systemic clearance from the blood stream via their uptake by the cells of mononuclear phagocyte system (MPS). As a result, they are most applied in macrophage targeting such as the delivery of antimicrobial agents to infected macrophages, local depot, vaccination and antigen delivery at present. The deficiency of conventional liposomes could be complemented by the addition of hydrophilic polymer (PEG) to enhance their stability and circulation time, therefore further their applications in nutrition, food and cosmetics industry, etc.
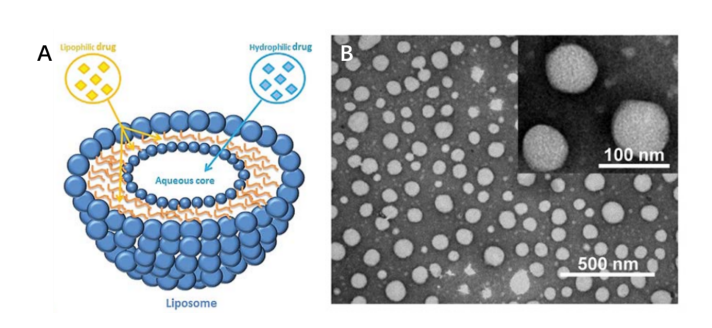
Figure 1. Conventional liposomes structure and size characterization by SEM.
Quotations and Ordering

References:
1. Tsermentseli S K, Kontogiannopoulos K N, Papageorgiou V P, et al. Comparative study of PEGylated and conventional liposomes as carriers for shikonin[J]. Fluids, 2018, 3(2): 36.
2. Navarro G, Cabral P, Cabrera M, et al. 99mTc-labeling and biological evaluation of conventional liposomes[J]. Alasbimn Journal, 2011, 13(51).
3. Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes a new frontier in cancer chemotherapy[J]. Tumori Journal, 2003, 89(3): 237-249.
4. Lila A S A, Ishida T. Liposomal delivery systems: design optimization and current applications[J]. Biological and Pharmaceutical Bulletin, 2017, 40(1): 1-10.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.