Nucleic acid aptamer (also known as nucleic acid recognizer) is a functional DNA structure that has been artificially synthesized and screened. It is a type of DNA or RNA sequence screened by exponentially enriched ligand system evolution (SELEX) technology. The sequence can form special three-dimensional structures (such as hairpins, rseudoknot, convex ring, etc.) by means of intermolecular forces such as hydrogen bonds, van der Waals forces, and hydrophobic interactions, so as to specifically and efficiently bind to various targets (such as metal ions, small molecules, proteins, cells, etc.). Nucleic acid aptamers and their in vitro screening techniques were first proposed by two research groups, Szostak and Gold. In 1990, the Gold research group used in vitro screening technology to obtain an oligoribonucleotide chain that specifically binds to T4DNA polymerase, and named the in vitro screening technology SELEX. In the same year, the Szostak research group also reported an RNA fragment that can bind to small-molecule organic dyes and defined it as a nucleic acid aptamer. The main process of nucleic acid aptamer screening technology is to first design and synthesize a random nucleotide library in vitro, then mix the nucleotide library with the target substance, and remove the nucleic acid sequence that is not bound to the target substance through a specific separation method. And then reverse screening the bound sequence to exclude non-specific binding nucleic acid sequence, and finally amplify the specific nucleic acid sequence by PCR technology until the dissociation constant of the obtained nucleic acid library to the target substance reaches the target value. The discovery of nucleic acid aptamers shows that nucleic acid molecules can not only serve as carriers of genetic information, but also interact with other molecules through their own specific structures.
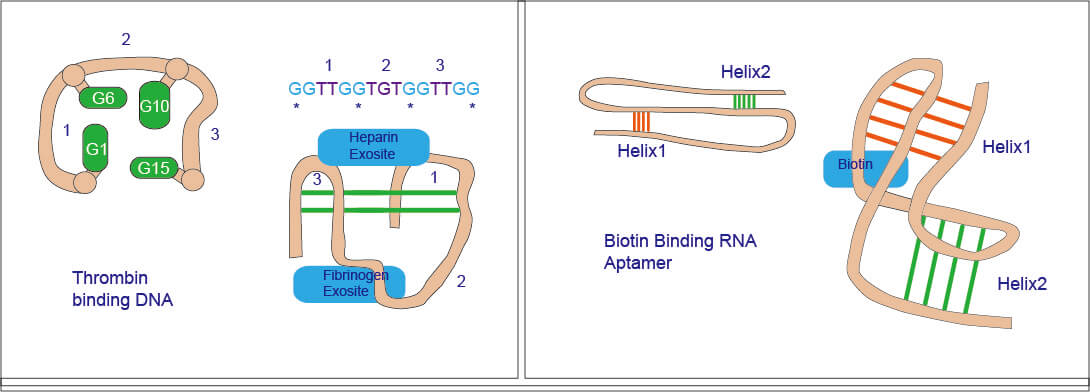
Figure 1. Schema diagram of Aptamer.
The detection of early cancer cells is essential for the clinical diagnosis and treatment of cancer. For a long time, the detection of cancer cells has mainly been judged by observing the morphology of cancerous tissues or cells, but this method cannot give an accurate diagnosis. The nucleic acid aptamers screened by Cel-SELEX technology can efficiently and specifically bind to specific cancer cells, and have important applications in the fields of cancer cell capture, detection and imaging, and accurately diagnose early cancers. The researchers combined nucleic acid aptamer-modified gold nanoparticles with microfluidic elements to develop a method for efficiently capturing and separating circulating tumor cells in the blood. Afterwards, the scientists designed an electrochemical sensor based on nucleic acid aptamers and functionalized graphene to detect cancer cells through the over-expressed nucleolin on the surface of cancer cells. In addition, some researchers have developed a nucleic acid aptamer with a hairpin structure that can specifically recognize cancer cells. When the targeted cancer cells are present, the hairpin structure opens to cause the fluorescent molecules it carries to generate signals, showing A fluorescence image of the distribution of cancer cells is displayed.
Using Cel-SELEX technology, multiple nucleic acid aptamers that bind to the corresponding cancer cells efficiently and specifically can be screened without knowing the molecular markers, which can be used for molecular-level targeted therapy and greatly improve the therapeutic effect of cancer, and reduce toxic side effects. Farkhzad and his collaborators were the first to use nucleic acid aptamers for targeted chemotherapy. They used polymer nanomaterials to load paclitaxel and modified them with nucleic acid aptamers. The complex was successfully used in simulated animal experiments, which significantly improved the therapeutic effect and reduced toxic. Afterwards, researchers attached polyethylene glycol ligands to the surface of hollow and porous magnetic nanoparticles, and then modified them with nucleic acid aptamers. This complex can simultaneously achieve targeted chemotherapy and MRI. In addition, in recent years, targeted photodynamic therapy and targeted photothermal therapy have also attracted people's attention. The highly selective nucleic acid aptamer is combined with a photosensitizer, and the photosensitizer can be excited to generate reactive oxygen species to kill cancer cells and achieve a targeted photodynamic therapy effect. Targeted photothermal therapy uses the surface plasmon resonance properties of gold and silver nanoparticles or nanorods. Under near-infrared light excitation, gold and silver nanomaterials can generate heat and kill specific cancer cell under the targeting action of nucleic acid aptamers.
A variety of drug delivery systems currently proposed are related to the treatment of cancer. It is well known that the absorption, release and site specificity of drugs play a key role in the drug delivery system. One of the most interesting is the delivery of drugs to specific targets, because this is a key factor in the effective display of drug effects in the body. Antibodies and nucleic acid aptamers are currently the most used ligands in targeted drug delivery systems. However, due to the high-efficiency specificity and high affinity of nucleic acid aptamers to target molecules, nucleic acid aptamers are considered to be the most powerful biological ligands. Nucleic acid aptamers that can bind to the intrinsic receptors on the cell surface have been used to transport drugs and other substances into the cell. For example, based on the expression of the specific membrane antigen (PSMA) of prostate cancer, prostate cancer cells can be divided into two cell lines, namely PSMA (+) and PSMA (-) cells. PSMA is a type II transmembrane protein on the glandular epithelial cell membrane of the prostate. It is specifically expressed in prostate cancer tissues. Therefore, PSMA is an important marker of prostate cancer. The LNCaP cell line in the experiment belongs to PSMA (+) cells, and the PC3 cell line belongs to PSMA (-) cells. A10 is an aptamer for PSMA (+) prostate cancer cells; DUP-1 is an aptamer for PSMA (-) prostate cancer cells. Combine doxorubicin (an anticancer drug) with the binary nucleic acid aptamer complex (A10 and DPU-1), and the combined doxorubicin can enter PSMA (+) or PSMA (-) Prostate cancer cells, which can effectively target doxorubicin to prostate cancer cells.
References:
1. Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990, 249(4968):505-10.
2. Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990, 346(6287): 818-22.
3. Wang L, et al.; Unmodified gold nanoparticles as a colorimetric probe for potassium DNA aptamers. Chem Commun (Camb). 2006, (36):3780-2.
4. Tan W, et al.; Molecular aptamers for drug delivery. Trends Biotechnol. 2011, 29(12):634-40.
5. Famulok M, et al.; Functional aptamers and aptazymes in biotechnology, diagnostics, and therapy. Chem Rev. 2007,107(9): 3715-43.
6. Sheng W, et al.; Multivalent DNA nanospheres for enhanced capture of cancer cells in microfluidic devices. ACS Nano. 2013, 7(8): 7067-76.
7. Feng L, et al.; A graphene functionalized electrochemical aptasensor for selective label-free detection of cancer cells. Biomaterials. 2011, 32(11): 2930-7.
8. Farokhzad OC, et al.; Targeted nanoparticle-aptamer bioconjugates for cancer chemotherapy in vivo. Proc Natl Acad Sci USA. 2006, 103(16):6315-20.
9. Nimjee SM, et al.; Aptamers: an emerging class of therapeutics. Annu Rev Med. 2005, 56: 555-583.
10. Min K, et al.; Dual-aptamer-baseddelivery vehicle of doxorubicin to both PSMA (+) and PSMA (-) prostate cancers. Biomaterials. 2011, 32 (8): 2124-2132.
11. Jinling Zhang, et al.; An ensemble of aptamers and antibodies for multivalent capture of cancer cells. Chem Commun (Camb). 2014, 50(51): 6722–6725.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.