Liposomes were first discovered and successfully prepared by Cambridge scientist Bangham in 1965. They are a unique microspherical structure that can encapsulate water-soluble drugs or fat-soluble drugs in lipid bilayers and hollow spaces. At the same time, as a drug carrier, liposomes can improve the solubility and stability of encapsulated drugs, and can also enhance the targeting effect of drugs, thereby reducing the occurrence of peripheral adverse drug reactions. Due to the strong invasiveness and destructiveness of tumors, anti-tumor research has always been a hot topic and a difficult topic. Liposomes have the characteristics of low immunogenicity, and can effectively utilize their high permeability and retention effect (EPR) at the tumor site, and through surface modification, they can produce a targeting effect on tumor cells.
Liposomes are microscopic vesicles with one or more aqueous chambers formed by a lipid bilayer, generally composed of phospholipids and cholesterol in a certain ratio. Phospholipids are used to form a bilayer structure, while cholesterol supports and maintains the bilayer structure. The two work together to improve the stability of liposomes. Liposomes have a unique core-shell structure, good biocompatibility and biodegradability, can protect sensitive drugs from environmental factors (light, temperature, pH, etc.), and improve the efficacy of drugs by controlling the release of drugs. According to the number of lipid bilayers, liposomes can be divided into unilamellar liposomes and multilamellar liposomes. Small unilamellar liposomes have a particle size of 20 to 100 nm and have good membrane stability, but low encapsulation rate and low drug loading; large unilamellar liposomes are large single-layer vesicles with high encapsulation rate for water-soluble drugs, particle size of 100 to 1 000 nm, high drug loading, but poor membrane stability; giant unilamellar liposomes have a particle size of >1 000 nm and almost no membrane tension. Vesicles with multiple bilayers are called multilamellar liposomes. The particle size of multilamellar liposomes is 8 to 12 μm. They have multiple lipid bilayers (usually more than 5 layers), good stability and high encapsulation rate, and can be used in the design and development of sustained-release preparations.
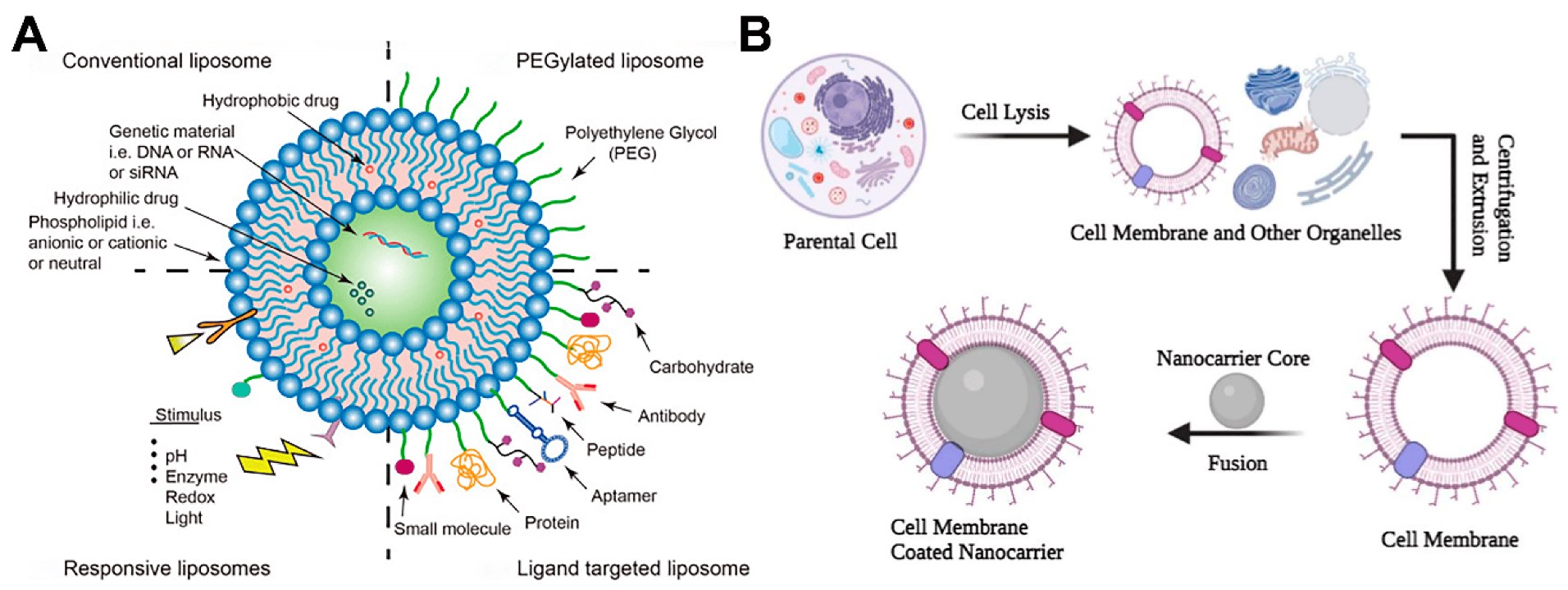 Figure 1. Schematic diagram of functionalized liposomes. (Wang S, et al.; 2023)
Figure 1. Schematic diagram of functionalized liposomes. (Wang S, et al.; 2023)
Passive Targeting Liposomes
Passive targeting means that liposomes can cause spontaneous retention based on the difference between the high permeability of tumor tissue and the microvascular structure of normal tissue, forming a natural tendency of enrichment. Traditional passive targeting liposomes are easily affected by substances such as laminin and complement proteins, and are thus recognized and taken up by macrophages, resulting in the rapid clearance of their contained drugs in the body. Therefore, long-circulating liposomes obtained by surface modification with polyethylene glycol (PEG), phosphatidylinositol, etc. are a major advance in the development of passively targeted liposomes. The liposome's metabolism rate in the body is significantly reduced, the drug's systemic circulation time is significantly prolonged, and the EPR effect is increased, making it easier for the drug to accumulate at the lesion site, reducing immune organ toxicity and adverse reactions. In the preparation process of long-circulation liposomes, PEG is the most widely used. As an extension of the hydrophilic end of long-circulating liposomes, PEG can increase steric hindrance, preventing plasma opsonized proteins from adhering and aggregating with liposomes, thereby protecting liposomes from being recognized in the blood, thereby reducing the risk of reticuloendothelial system intake.
Active Targeting Liposomes
Antibody-modified liposomes, also known as immunoliposomes, are liposomes that insert monoclonal antibodies or functional fragments into the surface of liposomes. The specific recognition ability of antibodies is used to enrich drugs in the target area, thereby reducing the toxic side effects of drugs and improving the efficacy. Based on the concept of precision medicine, antibody-modified liposomes are currently a hot area in the research of active targeting liposomes, including monoclonal antibodies, antibody Fab fragments, etc.
Due to some gene mutations in cancer cells, some receptors are overexpressed, while these receptors are less expressed or not expressed in normal cells. Based on the specific expression of tumors, liposomes are structurally modified with ligands or other functional groups through physical or chemical means to better play an active targeting role, thereby facilitating drug delivery.
Nucleic acid aptamers are single-stranded DNA or RNA oligonucleotides composed of 25 to 90 nucleotide bases that are synthesized through artificial screening. Nucleic acid aptamers can form specific three-dimensional spatial structures through secondary and tertiary structures, can accurately identify targets and play a targeting role, and have high specificity and strong affinity. Compared with traditional antibodies, nucleic acid aptamers have the advantages of target diversity, low molecular weight, and program controllability. They can effectively improve the ability of drugs to penetrate tumors and distribute evenly inside tumors. However, they also have shortcomings such as high development difficulty and lack of stable conformation.
Physical and Chemical Targeted Liposomes
Thermoresponsive materials (dipalmitoylphosphatidylcholine, distearoylphosphatidylcholine, etc.) are an indispensable component of thermosensitive liposomes. Drug release is mediated by temperature, and as the temperature rises, the physical properties of the lipid membrane change from a solid colloidal state to a highly permeable liquid crystal state, and the fluidity increases, causing the encapsulated drug to be released. Thermosensitive targeted liposomes are composed of special lipid substances with a phase transition temperature slightly higher than body temperature. During the treatment process, the target area is heated by an external heat source, causing the drug to aggregate at this heated site, thereby achieving the effect of targeted delivery.
Currently, pH-sensitive liposomes made of pH-sensitive materials such as dioleoylphosphatidylethanolamine can be delivered in a targeted manner through changes in pH. When the liposomes enter a weakly acidic or acidic local area, the carboxyl groups of the fatty acids in the pH-sensitive targeted liposomes are protonated, causing the cell membrane to fuse with the liposomes, which is beneficial for the delivery of drugs within the cells. Studies have shown that cancer cells produce a large amount of lactic acid through glycolysis, causing the pH of the tumor tissue environment to become acidic, and then use this feature to modify the liposomes to produce a targeting effect.
Reactive oxygen species (ROS) sensitive liposomes are prepared based on the high ROS concentration in tumor lesions. On the one hand, ROS can directly react with proteins, transcription factors, etc. to regulate the conduction pathway; on the other hand, ROS can oxidatively modify biological molecules and change their functions. ROS sensitive liposomes modify their lipids so that after reaching the lesion area, the lipid membrane structure is changed under the oxidative action of ROS, thereby releasing the encapsulated drugs.
Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.