Breast cancer is one of the most prevalent and deadly cancers globally, with metastasis being the leading cause of mortality among patients. Metastasis involves the spread of cancer cells from the primary tumor to distant organs such as the lungs, liver, bones, and brain. Notably, over 60% of breast cancer patients develop lung metastases in advanced stages. While surgery effectively treats primary tumors, late-stage metastases necessitate chemotherapy. However, despite advancements, the five-year survival rate for metastatic breast cancer remains low, approximately 20%, due to the severe off-target toxicity of conventional treatments. This highlights the urgent need for novel therapies offering high efficacy with minimal systemic side effects.
Developing targeted drug delivery systems is a promising approach to overcoming the limitations of traditional chemotherapy. Nanocarriers, including nanoparticles, micelles, and liposomes, are at the forefront of these innovations. Among them, liposomes are particularly advantageous due to their high drug-loading capacity and safety profile. Their unique structure, comprising a phospholipid bilayer and an aqueous core, allows for the co-encapsulation of both lipophilic and hydrophilic drugs, making them versatile and effective nanoscale drug delivery vehicles.
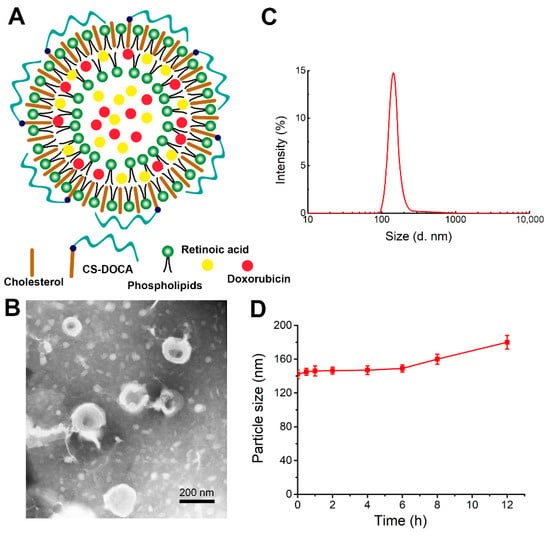 Figure 1. Characteristics of chondroitin sulfate modified liposomes. (Zhang Z, et al.; 2021)
Figure 1. Characteristics of chondroitin sulfate modified liposomes. (Zhang Z, et al.; 2021)
Despite their potential, nanocarriers face significant challenges, including poor stability, complex formulation procedures, and potential toxicity. Most nanocarriers, with diameters around 100 nm, rely on the Enhanced Permeation and Retention (EPR) effect to accumulate in highly vascularized primary tumors. Unfortunately, this approach is less effective for small metastatic tumors, which are often less vascularized, limiting the access of these nanocarriers.
Intratumoral injection, which enriches drugs directly within the tumor, has recently gained interest. When combined with immunotherapy, this approach can elicit a systemic anti-cancer response and address the challenges of poor drug targeting associated with intravenous administration. However, intratumoral injection is limited to superficial tumors and presents safety concerns, particularly regarding immunotherapy.
Innovative strategies are exploring the use of mucopolysaccharides such as hyaluronic acid, heparin and chondroitin sulphate (CS) to enhance the targeting ability of nanocarriers. These polysaccharides can bind to specific receptors on the surface of metastatic breast cancer cells, promoting targeted drug delivery to the lungs. Researchers have developed a system based on CS-modified liposomes for the co-delivery of doxorubicin (DOX) and retinoic acid (RA) to inhibit breast cancer lung metastasis.
DOX is a well-established chemotherapeutic agent used to treat various cancers, including breast cancer metastasis. It works by inserting itself into DNA, inhibiting the replication of cancer cells. RA is known for its ability to inhibit cancer cell proliferation and disrupt the Golgi apparatus. RA also exhibits lower toxicity and higher in vivo stability compared to other Golgi disruptors, making it an ideal candidate for combination therapy with DOX.
Nanodrug carrier systems consisting of CS-modified liposomes loaded with DOX and RA (called DOX+RA-CSL) were evaluated for their drug delivery and uptake efficiency. They were compared with CS-free liposomes and single drug-loaded liposomes in vitro and in vivo. The antitumour efficacy of DOX+RA-CSL was also evaluated in a mouse lung metastasis model of breast cancer.The study systematically evaluated the drug delivery and uptake efficiency of CS-modified liposomes co-loaded with DOX and RA, referred to as DOX+RA-CSLs. These were compared with CS-free liposomes and single drug-loaded liposomes both in vitro and in vivo. The antitumor efficacy of DOX+RA-CSLs was also assessed using a murine model of breast cancer lung metastasis.
The development of chondroitin sulfate-modified liposomes for the targeted co-delivery of doxorubicin and retinoic acid represents a significant advancement in treating breast cancer lung metastasis. By enhancing cellular uptake, and stability, and reducing systemic toxicity, this multifunctional drug delivery system offers a promising strategy for combination cancer therapy. The positive results from both in vitro and in vivo studies suggest that DOX+RA-CSLs could serve as an effective and simple drug delivery system, potentially improving the survival and quality of life for patients with metastatic breast cancer. Further research and clinical trials are warranted to fully realize the therapeutic potential of this innovative approach.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.