Glucan consists of glucose residues linked by α-1,6 glycosidic bonds to form the main chain, and a small amount of α-1,2, α-1,3, α-1,4 glycosidic bonds forming the side chain. The molecular formula is (C6H10O5)n. As a natural polysaccharide, dextran has a simple structure, good water solubility, high biocompatibility and is non-toxic and harmless to the body. Its application in the field of drug delivery has become one of the research hotspots that has attracted much attention in recent years. Drug carriers are systems that can affect the delivery, distribution and release of drugs in the body and precisely transport them to target organs. The drugs they contain are usually anti-tumor drugs to overcome problems such as high toxicity, poor water solubility, short half-life, easy degradation in the body and lack of specific targets. The surface of the dextran molecular structure contains many active hydroxyl groups which can be chemically modified to form different drug carrier forms such as nanomicelles, nanogels and prodrugs. This article focuses on a more comprehensive review of the research progress in the synthesis methods of functionalized dextran derivatives for drug carriers from the perspective of chemical macromolecular structure modification, and proposes the issues that urgently need to be solved in the synthesis and preparation research methods and drug carrier applications of dextran derivatives. problem, providing a useful reference for the research on biological applications of new glucan derivatives in pharmaceutical carriers and other fields.
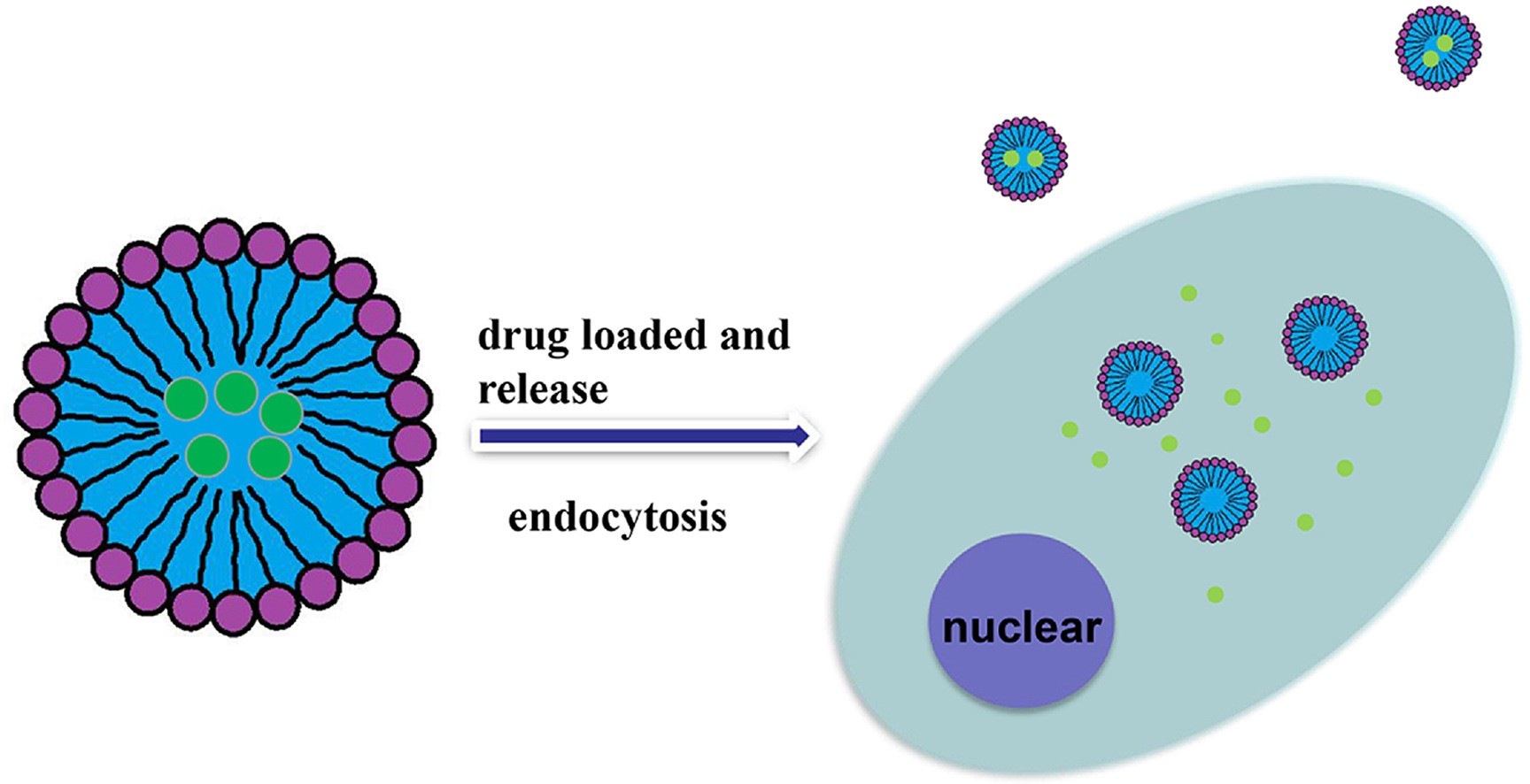 Figure 1. Design and application of dextran carrier. (Shiyu Huang, et al.; 2020)
Figure 1. Design and application of dextran carrier. (Shiyu Huang, et al.; 2020)
The oxidation of dextran using IO4- is a non-catalytic reaction in aqueous solution. Research began in 1928 with the aim of determining the structure of the polysaccharide by analyzing the oxidation products of dextran. In recent years, research on oxidised dextran has focused on the use of mild oxidation conditions to provide aldehyde side chains to dextran. First, an I-O bond of IO4- attacks a hydroxyl group of the vicinal diol to break the C-C bond; then excess IO4- completely oxidises the remaining hydroxyl groups in dextran, consuming 2 moles of IO4- to form a dialdehyde structure. The active aldehyde group of the oxidised dextran side chain has the property of undergoing nucleophilic addition reaction with nucleophiles and can undergo dehydration condensation with nucleophiles such as amines and hydrazines to form Schiff bases or acylhydrazone Schiff bases containing imino (HC = N) or alkylimino structures. Oxidised dextran Schiff base derivatives are mainly used in pharmaceutical research for drug delivery or sustained release, and their reactions fall into two main categories.
Oxidised dextran can react directly with the amino groups of amine compounds to form Schiff bases. Some researchers have used chitosan amino groups and oxidised dextran aldehyde groups to condense into Schiff bases, which are cross-linked into a hydrogel network structure that has good antibacterial properties and can prevent and control post-operative infections. Oxidised dextran can also undergo condensation reactions with amino-containing proteins, improving protein bioavailability without altering protein activity and conferring new properties. Sodium cyanoborohydride can be used as a reducing agent for reduction and amination reactions to create a stable amino structure between protein and dextran. Currently, proteins or enzymes that have been successfully coupled to oxidised dextran include alpha-amylase, glycerol dehydrogenase, serum protein, etc.
The aldehyde group of oxidised dextran can condensate with the hydrazine group of the hydrazide to form an acylhydrazone Schiff base compound with a -CONHN = CH- structure. At this point, the acyl group attached to the imino double bond and the secondary amino group form a p-π conjugation, making the structure more stable. Some researchers have condensed oxidised dextran with adipic acid dihydrazide (AAD) to form an acylhydrazone Schiff base, and the resulting hydrogel can be used in ophthalmic surgery or injectable drugs.
Studies have found that dextran can react with compounds with hydrophobic carboxylic acid groups to form amphiphilic polymers, which can then be self-assembled to obtain a new type of drug micelle carrier. The micelle has a hydrophilic dextran shell, which allows it to avoid recognition and phagocytosis by the reticuloendothelial system (RES), prolonging the drug's circulation time in the body; at the same time, it also has a hydrophobic drug storage core and has a strong drug loading capacity. In addition, dextran can directly form esters with carboxyl drugs to transport them into the body. However, unlike the classical esterification reaction, dextran has a large molecular weight and a relatively complex structure. When the esterification reaction occurs, steric hindrance will limit the reaction. Therefore, there are usually two pathways to accelerate the dextran esterification process: (1) using a coupling agent to promote the esterification reaction; (2) chlorinating the carboxyl-containing reactant and then esterifying it with dextran.
Common coupling agents include dicyclohexylcarbodiimide (DCC), 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N, N'-carbonyldiimidazole (CDI). The coupling mechanism is similar (taking DCC as an example), the carboxylic acid reactant first reacts with DCC to form an intermediate O-acylurea, and then forms an ester with the hydroxyl group of dextran.
Acyl chloride reacts with carboxylic acid compounds to form an acyl halide, which can then form an ester with the hydroxyl group of dextran.
According to the reductive amination mechanism, sodium cyanoborohydride (NaCH3BN) is used as a reducing agent, and the amino compound reacts with the terminal aldehyde group of dextran to graft various structural groups to the end of dextran, thereby achieving the modification of dextran.
Dextran and chloroacetic acid can undergo carboxymethylation reaction under strong alkaline conditions. Its products can be used in many fields such as pharmaceuticals, food, and cosmetics. Carboxymethyl dextran can be used to embed magnetic nanoparticles, that is, a new type of drug carrier with dextran as an organic macromolecular shell and an inorganic magnetic material as the core. This carrier shell has high biocompatibility and carries active groups on the surface, providing structural guarantees for carrying macromolecular compounds such as anticancer drugs and antibodies. A study used carboxymethyl dextran to embed iron oxide magnetic nanoparticles and found that compared with natural dextran-embedded nanoparticles, carboxymethylation can make the nanoparticles obtain better dispersibility and colloidal stability. After the active carboxyl groups are grafted onto the surface of carboxymethyl dextran, different dextran derivatives can be further modified.
The exposed hydroxyl groups on the surface of dextran in a strong alkaline solvent medium can act as nucleophiles to promote the ring opening of epoxy ethers, and various functional groups are connected to the surface of dextran, thereby changing the physical, chemical and biological properties of dextran. Dextran reacts with epoxyalkyl groups, and its surface is grafted with alkyl functional groups of different lengths. The water solubility of the polymer can be controlled according to the length and number of the alkyl groups. For example, dextran reacts with epoxyhexane and epoxydodecane with OH- as a catalyst in a dimethyl sulfoxide solvent to generate a polymer, and the degree of substitution of the polymer can be controlled by adjusting the proportion of epoxy compounds. Studies have shown that the greater the degree of substitution, the worse the water solubility of the resulting polymer, that is, the polymer has amphiphilic properties. Dextran can also undergo etherification reactions with aromatic epoxyalkyl groups (such as 1,2-epoxy-3-phenoxypropane) to achieve dextran modification.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.