Resveratrol is a natural compound with a wide range of biological activities, including antioxidant, anti-inflammatory, anti-tumor, anti-aging, and neuroprotective and cardioprotective effects. However, resveratrol has low water solubility, resulting in poor oral absorption and poor chemical stability. Therefore, finding effective delivery systems to overcome solubility, stability and bioavailability issues is a major challenge. Liposomes, as one of the nanocarriers, have been successfully incorporated with resveratrol to improve its bioactivity and stability under UV irradiation.
This encapsulation property of liposomes makes them an effective drug delivery system to enhance the absorption and distribution of resveratrol in the body and to enhance its pharmacological effects while reducing potential side effects. CD Bioparticles has extensive experience in developing liposomes over the years and has the capability for stable batch production and industrialization. Liposomal resveratrol prepared using high-pressure homogenization technology exhibit the following properties:
| Resveratrol Content | Phospholipid Content | Phospholipids | Encapsulation Rate | Particle Size (DLS) |
|---|---|---|---|---|
| 5%, 50% | 10-50% | Sunflower Phospholipid (P2) | 20-40% | 45 nm |
Note: P2 is a special process formulation 1. P6 is a special process formulation 2.
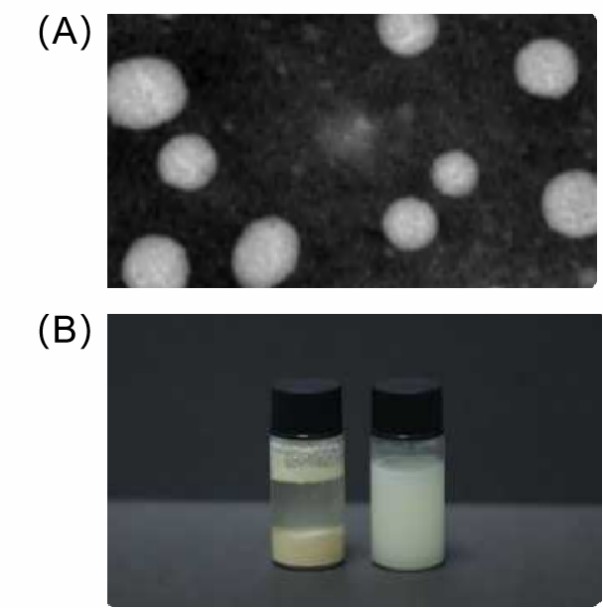 Figure 1. (A) TEM picture of liposomal resveratrol; (B) Plain resveratrol on the left and liposomal resveratrol on the right.
Figure 1. (A) TEM picture of liposomal resveratrol; (B) Plain resveratrol on the left and liposomal resveratrol on the right.
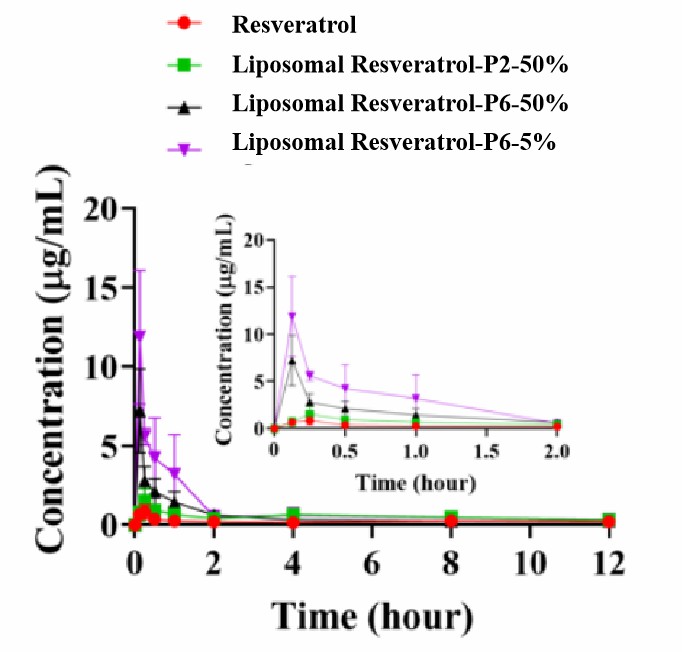 Figure 2. Pictures of blood concentrations of Liposomal Resveratrol.
Figure 2. Pictures of blood concentrations of Liposomal Resveratrol.
Table 1 Blood concentration analysis of resveratrol in different groups.
| Parameter | Resveratrol | Liposomal Resveratrol P2-50% | Liposomal Resveratrol P6-50% | Liposomal Resveratrol P6-5% |
|---|---|---|---|---|
| Cmax (μg/mL) | 0.83 ± 0.45 | 1.53 ± 0.77 | 7.21 ± 2.65 | 11.88 ± 4.23 |
| AUC0-t (μg/mL·h) | 2.8 ± 1.67 | 6.43 ± 1.97 | 6.74 ± 2.86 | 11.76 ± 6.51 |
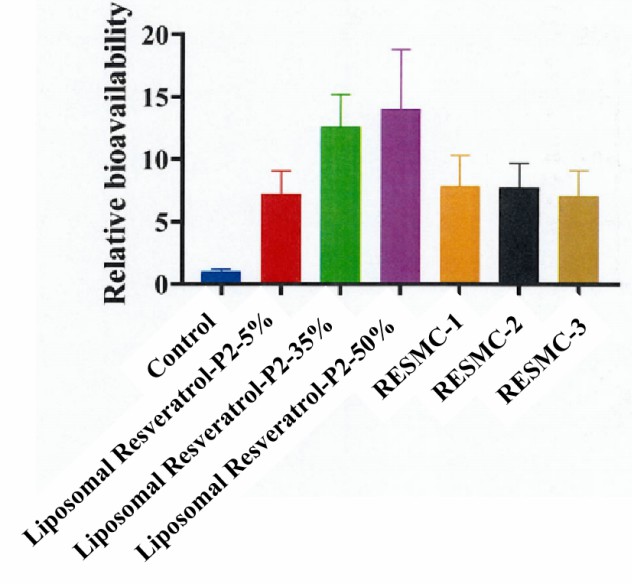 Figure 3. Pictures of relative bioavailability of liposomal resveratrol.
Figure 3. Pictures of relative bioavailability of liposomal resveratrol.
Table 2 Relative bioavailability of resveratrol in different groups.
| Parameter | Control | Liposomal Resveratrol P2-5% | Liposomal Resveratrol P2-35% | Liposomal Resveratrol P2-50% | RESMC-1 | RESMC-2 | RESMC-3 |
|---|---|---|---|---|---|---|---|
| AUC0-12h (ug/mL·h) | 6.38 ± 0.93 | 4.6 ± 1.17 | 8.04 ± 1.64 | 8.94 ± 3.03 | 5.03 ± 1.55 | 4.95 ± 1.21 | 4.51 ± 1.30 |
| RB | 1 ± 0.15 | 7.21 ± 1.83 | 12.6 ± 2.57 | 14.02 ± 4.75 | 7.88 ± 2.43 | 7.75 ± 1.90 | 7.07 ± 2.04 |

Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.