Liposomal Silymarin-Case Study
Background
Silymarin has significant antioxidant, hepatoprotective, anticancer and neuroprotective pharmacological effects. However, silymarin is poorly soluble in water and fat, resulting in inefficient drug delivery, which limits its clinical application. Higher dosages and more frequent administration are often required to achieve therapeutic effects. In recent years, silymarin liposomes have shown excellent results in both in vitro and in vivo models, effectively alleviating lipid metabolism disorders, insulin resistance and inflammation. Liposome technology encapsulating silymarin not only reduces toxicity but also prolongs the therapeutic effect.
Challenge
- Low solubility & Low bioavailability
- Chemical stability issues
- Biological stability issues
- Imprecise controlled release technology
Solution
This encapsulation property of liposomes makes them an effective drug delivery system that can improve drug stability and bioavailability while reducing toxic side effects and enabling targeted delivery. CD Bioparticles has extensive experience in developing liposomes over the years and has the capability for stable batch production and industrialization. Liposomal silymarin prepared using high-pressure homogenization technology exhibit the following properties:
Results
| Silymarin Content | Phospholipid Content | Phospholipids | Encapsulation Rate | Particle Size (DLS) |
|---|
| 30% | 10-50% | Sunflower Phospholipid (P2) | 20-40% | 28 nm |
Note: P2 is a special process formulation 1. P6 is a special process formulation 2.
- As shown in Figure 1 , the experimental results indicate that liposomal silymarin are spherical in shape and homogeneous, and the liposome nanosize is around 28 nm. The encapsulation rate was 20-40%. As can be seen from Figure 1B, ordinary silymarin is difficult to dissolve in water and shows obvious stratification, while liposomal silymarin can form a homogeneous and stable system in water and possesses better utilization.
 Figure 1. (A) TEM picture of liposomal silymarin; (B) Plain silymarin on the left and liposomal silymarin on the right.
Figure 1. (A) TEM picture of liposomal silymarin; (B) Plain silymarin on the left and liposomal silymarin on the right.
- The maximum blood concentration Cmax of liposomal silymarin-P2 and liposomal silymarin-P6 were 1.41 μg/mL and 1.69 μg/mL, respectively, which were much higher than that of the silymarin original group (0.74 μg/mL). Meanwhile, the area of drug-time curves AUC of the two groups, were 1.82 and 1.87 times higher than that of the silymarin original group, respectively. It indicated that the bioavailability of silymarin was significantly increased after it was prepared as a liposome, the bioavailability of silymarin was significantly increased. (Figure 2)
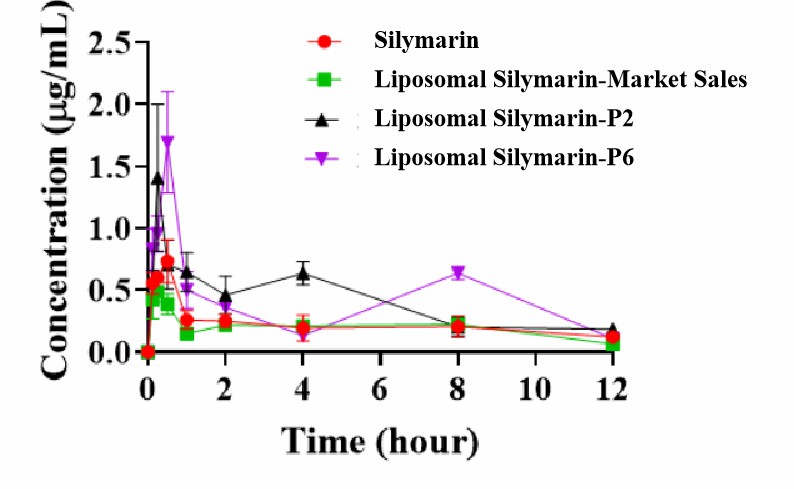 Figure 2. Pictures of blood concentrations of liposomal silymarin.
Figure 2. Pictures of blood concentrations of liposomal silymarin.
- CD Bioparticles sells two liposomes with enhanced free radical scavenging and iron ion reduction capabilities, which have significantly greater antioxidant capacity than liposomal silymarin-market sales. Compared to silymarin API, these two liposomes have a higher dissolution rate and solubility, and thus greater antioxidant capacity. It can be inferred that CD Bioparticles' own two liposomes will be more effective in liver protection compared to silymarin API and liposomal silymarin-market sales. (Figure 3)
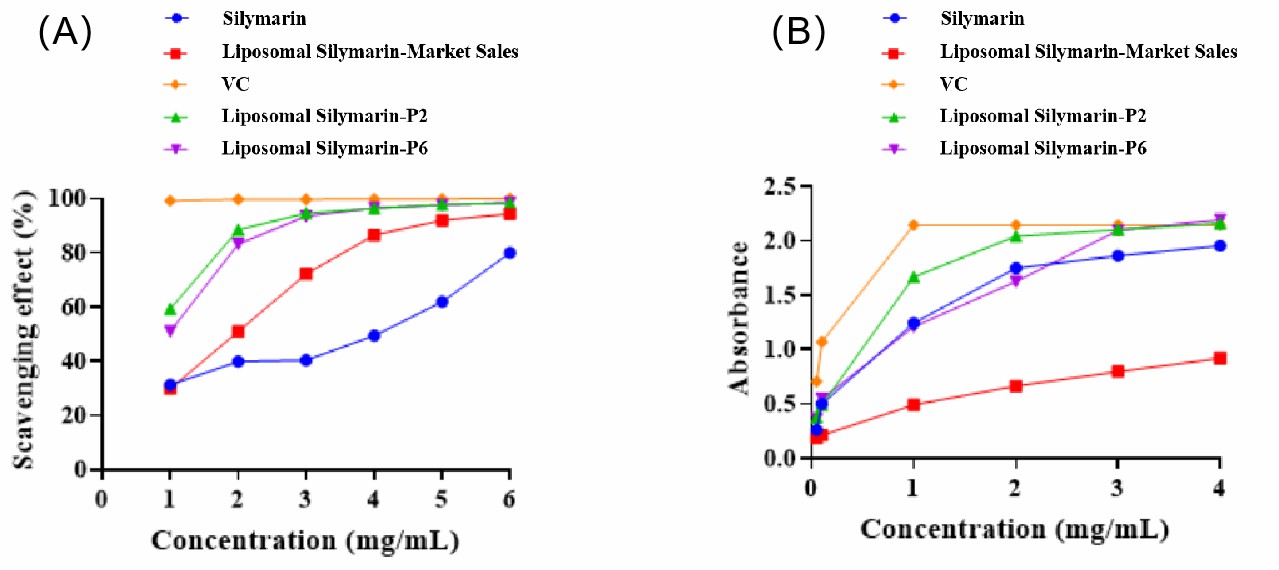 Figure 3. (A) Scavenging capacity of silymarin for hydroxyl radicals (B) Reducing capacity of silymarin for iron ions.
Figure 3. (A) Scavenging capacity of silymarin for hydroxyl radicals (B) Reducing capacity of silymarin for iron ions.
- As can be seen from the table 2, the AUC0-12h of all four groups of silymarin liposomes was higher than that of the silymarin prodrug group. Among them, the highest RB value of liposomal silymarin-P2-30% reached nearly 50-fold, indicating that the bioavailability of silymarin was significantly increased after it was prepared into liposomes. This improvement may be related to the phospholipids in the liposomes of silymarin, which are an important part of the cell membrane and can maintain the fluidity of the cell membrane, thus improving the absorption of the drug by the mucous membrane of the gastrointestinal tract. It may also be that the average particle size of silymarin decreases after it is prepared into liposomes, the specific surface area increases, and the solubility increases in water, which elevates the chance of contact with the intestinal tract, and it is easier to be absorbed. From the RB values in the table, it can also be seen that the in vivo absorption of the prepared silymarin liposomes (liposomal silymarin-P2-30% and liposomal silymarin-P6-30%) was significantly better than that of the commercially available silymarin phospholipid complex grains.
Table 1 Blood concentration analysis of silymarin in different groups.
| Parameter | Silymarin | Liposomal Silymarin -Market Sales | Liposomal Silymarin -P2 | Liposomal Silymarin -P6 |
|---|
| Cmax (μg/mL) | 0.74 | 0.49 | 1.41 | 1.69 |
| Tmax (h) | 0.5 | 0.25 | 0.25 | 0.25 |
| AUC0-t (μg/mL·h) | 2.67 | 2.39 | 4.86 | 5.00 |
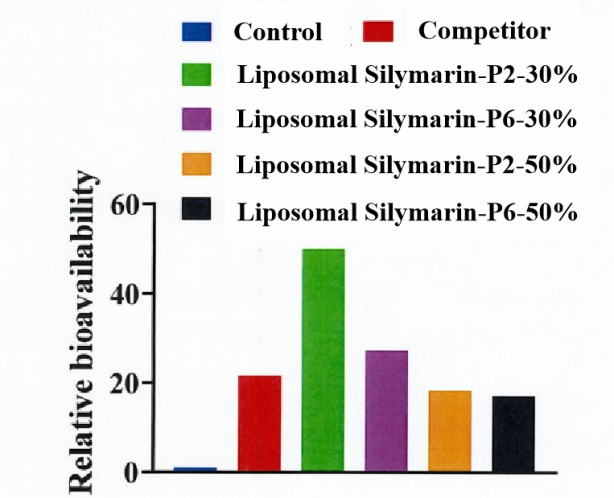 Figure 4. Pictures of relative bioavailability of liposomal silymarin.
Figure 4. Pictures of relative bioavailability of liposomal silymarin.
Table 2 Relative bioavailability of silymarin in different groups.
| Parameter | Control | Competitor | Liposomal Silymarin P2-30% | Liposomal Silymarin P6-30% | Liposomal Silymarin P2-50% | Liposomal Silymarin P6-50% |
|---|
| AUC0-12h (ug/mL·h) | 1.128 | 2.607 | 5.632 | 3.071 | 2.06 | 1.928 |
| RB | 1 | 21.57 | 49.93 | 27.23 | 18.26 | 17.09 |
Methods

Reference
- Kumar N, et al.; Improved in vitro and in vivo hepatoprotective effects of liposomal silymarin in alcohol-induced hepatotoxicity in Wistar rats. Pharmacological Reports. 2019, 71(4): 703-712.
 Figure 1. (A) TEM picture of liposomal silymarin; (B) Plain silymarin on the left and liposomal silymarin on the right.
Figure 1. (A) TEM picture of liposomal silymarin; (B) Plain silymarin on the left and liposomal silymarin on the right. Figure 2. Pictures of blood concentrations of liposomal silymarin.
Figure 2. Pictures of blood concentrations of liposomal silymarin. Figure 3. (A) Scavenging capacity of silymarin for hydroxyl radicals (B) Reducing capacity of silymarin for iron ions.
Figure 3. (A) Scavenging capacity of silymarin for hydroxyl radicals (B) Reducing capacity of silymarin for iron ions. Figure 4. Pictures of relative bioavailability of liposomal silymarin.
Figure 4. Pictures of relative bioavailability of liposomal silymarin.