Minocycline Hydrochloride (MH) is a semi-synthetic tetracycline broad-spectrum antibiotic with high efficiency and long duration of action. With the aging of the population, rising incidence of chronic diseases and other social problems, the demand for long-lasting, safe and convenient drug formulations is increasing. Slow-release microsphere/microcrystalline formulations, as an advanced form of drug preparation, have a broad market prospect. Microspheres/microcrystalline sustained-release formulations are administered in various ways, including orally, subcutaneously implanted, intramuscularly injected, intravenously injected and so on. Among them, the diameter of microspheres for injection is generally controlled to be around 100 micrometers, so as to facilitate the entry into the human body through the syringe. Minocycline Hydrochloride extended-release microspheres can prolong the duration of action of the drug in the body, reduce the number of times the patient has to give the drug, and improve the convenience of treatment and patient compliance . In addition, it can improve the solubility and bioavailability of the drug so that it can be absorbed and utilized more effectively in the body. Since microsphere formulations can reduce the systemic distribution of drugs, they can reduce the impact of drugs on the systemic system and reduce the risk of bacterial resistance.
Ensuring that microspheres maintain the stability of their structure and release properties during storage and use is critical to the commercialization and clinical application of long-acting microspheres.
Utilizing our microsphere sustained-release platform, we can provide microsphere sustained-release formulations to extend the duration of drug action. In addition, we can ensure the industrialization of microspheres on the ground with a production capacity, and can help customers to do the FDA registration.
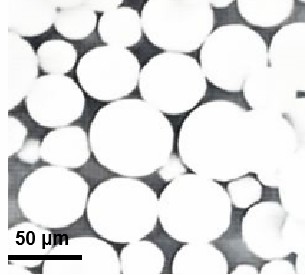 Figure 1. TEM picture of drug-loaded microspheres for injection.
Figure 1. TEM picture of drug-loaded microspheres for injection.
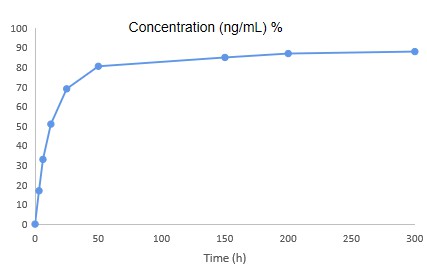 Figure 2. Pictures of blood concentrations of minocycline hydrochloride microspheres.
Figure 2. Pictures of blood concentrations of minocycline hydrochloride microspheres.
Table 1 Comparative experimental results of various formulations of minocycline hydrochloride in rats.
| Summary of pharmacokinetic parameters | |||||||
|---|---|---|---|---|---|---|---|
| Group | T1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUC(0-t) (h*ng/mL) | AUC(0-∞) (h*ng/mL) | MRT (0-t)(h) | MRT (0-∞)(h) |
| Minocyclinc hydrochloride microspheres once a week | 174.795 | 10.655 | 660 | 27016 | 31423.5 | 57.22 | 130.42 |
| Minocyclinc hydrochloride Sustained-release tablets (once daily) | 14.255 | 0.83 | 1586.5 | 7984.5 | 8138 | 5.905 | 7.51 |
| Minocyclinc hydrochloride capsules(three times daily) | 5.105 | 1.25 | 1892 | 8990 | 9049.5 | 4.1 | 4.36 |

Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.