Metal-organic frameworks (MOFs) are coordination compounds formed by metal ions or ion clusters linked to organic ligands, with regular lattices, large specific surface areas and well-controlled pores. As a new type of porous material, MOFs have the advantages of large specific surface area, high porosity, easy surface modification, high drug loading rate and good biodegradability. At present, MOFs with zinc, iron and copper metal cores have been extensively studied in drug loading and are used in disease treatment, anti- tumor, etc., with good application prospects. This article summarizes the types of MOFs commonly used for drug loading and their targeted release mechanisms based on the research progress in recent years.
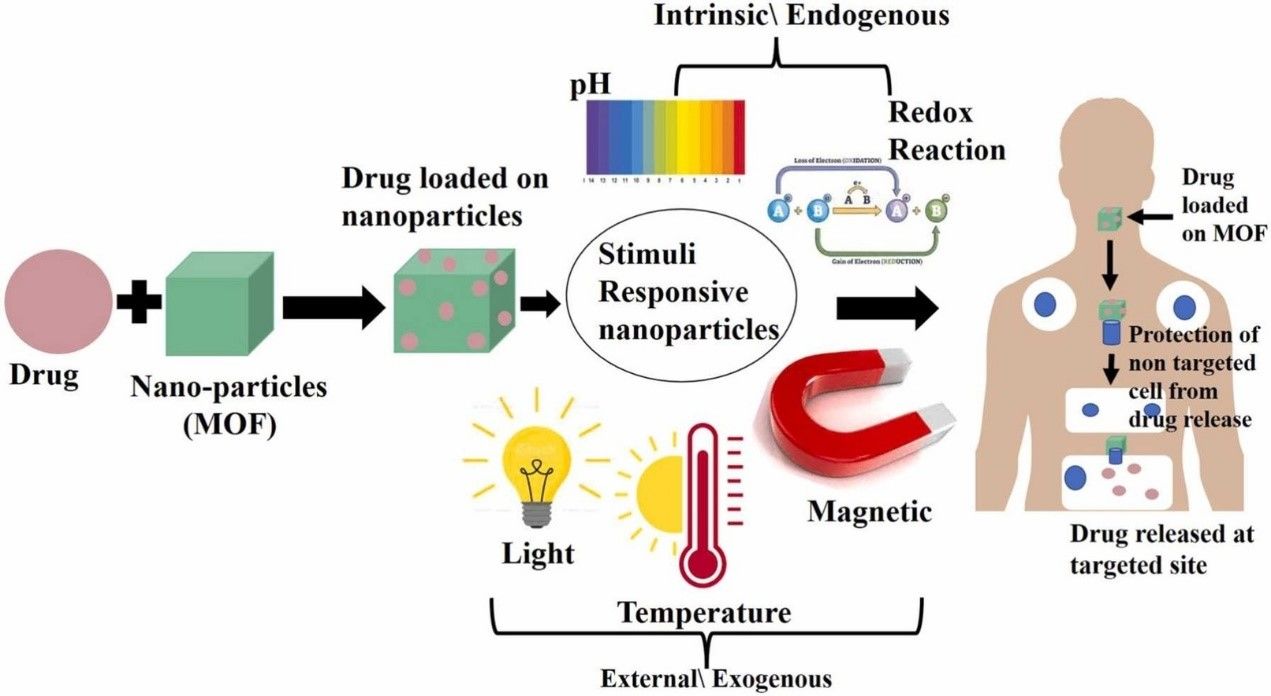 Figure 1. Metal-organic frameworks for targeted drug delivery. (Sanjeev Gautam, et al.; 2023)
Figure 1. Metal-organic frameworks for targeted drug delivery. (Sanjeev Gautam, et al.; 2023)
Zeolite imidazolate framework (ZIF-8) is a porous material composed of zinc ions (Zn2+) and 2-methylimidazole (MelM) coordinated and self-assembled. ZIF-8 has significant drug loading (can load tetracycline, metformin, insulin, etc.), biocompatibility and acidic environment sensitivity. It has good stability under physiological conditions, but is easy to disintegrate under acidic conditions, which makes It is responsive to the weakly acidic environment associated with various diseases (such as malignant tumors), making it an ideal carrier for controlled drug transport and release.
MIL series materials (MIL) can be constructed with trivalent metals. Currently, MOFs constructed with iron ions are mainly used for drug loading. MIL-100(Fe) is a material composed of Fe, 1,3,5-benzenetricarboxylic acid, hydrofluoric acid, nitric acid and water mixed in a certain molar ratio. It has a dual-pore structure and biocompatibility, hydrothermal stability, acidic environment sensitivity and amphiphilic internal microenvironment (hydrophilic inorganic part and hydrophobic inorganic part), which have potential application value in the field of biomedical applications. The acidic environment sensitivity of MIL-100(Fe) is also suitable for pH-responsive delivery systems. At the same time, the hydrothermal stability and amphiphilic internal microenvironment of MIL-100(Fe) enable it to have a wide range of drug loading properties and can be loaded Drugs such as zinc phthalocyanine, doxorubicin (DOX), and docetaxel can also decompose H2O2 through Fenton-like reactions in the tumor environment to supply O2, so they are widely used in photothermal therapy for cancer. In recent years, some new MIL-100(Fe) has been reported to be used for drug delivery, such as PCN-250 (Fe).
Cu-MOF is a porous material formed by self-assembly of copper ions (Cu2+) and organic ligands. Cu-MOF has significant drug loading capabilities (can load ibuprofen, methotrexate, disulfiram, etc.) and biocompatibility, and can also react with strong reducing agents to achieve redox response.
The above three materials have their own characteristics, as well as relative advantages and disadvantages due to different synthetic raw materials and methods. These factors should be taken into consideration and selected in the actual selection of drug carriers to achieve the ideal drug transportation effect. Comparing the characteristics of the above three materials, it was found that the ZIF-8 material is relatively cheap, the synthesis method is relatively simple, and can be synthesized at room temperature. As a drug carrier, it has significant drug loading, biocompatibility, stability and acidic environment. Sensitivity and responsiveness to the weakly acidic environment associated with various diseases; MIL-100 (Fe) material cost is higher than ZIF-8. It is mainly prepared by the solvothermal method. The synthesis conditions are more stringent than ZIF-8. As a drug carrier, in addition to being biocompatible and sensitive to acidic environments, the carrier also has hydrothermal stability and an amphiphilic internal microenvironment. It can load a variety of drugs and can also be used for tumor photothermal therapy through Fenton-like reactions in the tumor environment; Cu-MOF varies according to the selection cost and synthesis method of organic ligands. As a drug carrier, it has significant drug loading and biocompatibility, and can also undergo oxidation and reduction with strong reducing agents to achieve responsive release.
The binding of receptors to ligands is highly specific. In the body, the number and type of receptors on the surface of different cells differ. This specific recognition not only plays an important role in information transfer, but also provides new ideas for targeted drug delivery. Through functionalisation, the corresponding ligands of the targeted cells are attached to MOFs. After entering the body, the MOF ligand is specifically recognised by the targeted cells, reducing toxic effects on normal cells and achieving targeted drug delivery. Folate receptors (FR) are often expressed on the surface of cancer cells, and FR has a good affinity for folic acid (FA), which is ideal for targeted recognition.
High Permeability and Long Retention (EPR) refers to the property that some molecules or particles of certain sizes (such as liposomes, nanoparticles, etc.) are easier to penetrate into tumor tissues and stay there for a long time compared to normal tissues. This property of tumor cells provides a potential avenue for targeted delivery of anticancer drugs. Nano-sized MOF materials can exploit this property to achieve accumulation in tumor cells.
The physicochemical properties of some MOFs make it possible to use exogenous conditions to target drug delivery systems. Currently, the most widely used method is to target magnetic MOFs to specific tissues by applying an external magnetic field.
pH Response
Under normal circumstances, the internal environment of the body maintains a slightly alkaline pH environment, while the pH in tumor tissues, lysosomes and endosomes is generally low. In view of this tissue characteristic, some acid-sensitive drug delivery systems can be used for pH-responsive drug release. The mechanism of pH-responsive drug release can be mainly divided into the following types: (1) Protonation-induced covalent bond cleavage, which causes the MOFs structure to disintegrate or the connecting bonds to break through the change in pH to achieve the purpose of drug release. The former is because some MOFs materials contain ionizable organic ligands (such as imidazole, amino, carboxyl, pyridyl), which remain deprotonated in a physiological weak alkaline environment. When the pH drops, protonation causes the coordination bonds between metal ions and organic ligands to break, and the MOFs material disintegrates to release the drug; the latter uses the acid instability of the covalent bonds (such as ether, hydrazine, amide, etc.) connecting some drugs to MOFs. When the pH drops, the covalent bonds are hydrolyzed to release the drug. (2) Reversal of electrostatic action. Due to pH changes, the charge properties of drug molecules loaded onto MOFs by electrostatic adsorption change, and the originally mutually attractive electrostatic effect becomes a repulsive effect to release drugs, such as tinidazole, 5-fluorouracil, etc. (3) pH-sensitive materials as gated trigger devices. By adding a layer of pH-sensitive material to the outside of MOFs, it is cleaved under acidic conditions to release the internal drugs.
Redox Response
The purpose of drug release is achieved by breaking the connecting bonds or disintegrating the MOFs structure through redox. Tumor tissue has a high concentration of glutathione (GSH), which can undergo redox reactions to cause bond breaking or metal ion reduction, causing MOFs to cleave and drug release. It is also possible to release drugs by causing redox reactions at specific tissue locations through enzyme loading, such as hyaluronidase/hyaluronic acid; the loading of glucose oxidase (GOD) is often used to control insulin release and achieve response to glucose. GOD is used to convert glucose into gluconic acid and H2O2, causing local acidification, further triggering the pH response mechanism to achieve drug release.
Coordination Competition Response
The original coordination structure is destroyed by the combination of ionic organic ligands with stronger coordination ability, resulting in the cleavage of MOFs to release drugs. The lone pair electrons of the N atom in adenosine triphosphate (ATP) are rich and have strong coordination ability. They can compete with the original organic ligands in MOFs to destroy the original structure.
Nucleic Acid Cap Lock Response
After encapsulating the drug into MOFs, a layer of nucleic acid cap lock structure is loaded on the surface of MOFs to prevent drug leakage. When encountering a response substance, the nucleic acid combines with the response substance to form a special complex that falls off the surface of MOFs to achieve the purpose of releasing the drug. There are mainly ATP-sensitive and ion-sensitive cap lock structures.
The purpose of drug release is achieved by giving energy through exogenous artificial additional conditions to break the connecting bonds or disintegrate the MOFs structure. Currently, the commonly used exogenous conditions are light, heat and ultrasound. Photoresponse achieves the purpose of controlling drug release through conformational changes, chemical bond breaking or photothermal conversion of materials under light. Some special photosensitive molecules can be used as organic ligands to design photoresponsive MOFs, which are widely used in photodynamic therapy of tumors. For example, azophthalate undergoes isomerization under light irradiation, resulting in MOFs cleavage; porphyrin can produce strong oxidizing substances (such as O2) under light irradiation to destroy the connecting chemical bonds, resulting in drug release. Thermal response releases drugs by destroying the intermolecular interaction force caused by high temperature environment.
The specificity of only one response mechanism may not be ideal due to fewer restrictions. A higher specificity of drug release response can be achieved by combining multiple response mechanisms. Generally speaking, exogenous stimuli often cooperate with endogenous stimuli to achieve the purpose of stimulus-responsive drug release. The multiple response mechanism of pH coordinating with exogenous stimuli is the most common. Among them, the pH/light dual response mechanism is the most common and is often used in photothermal-chemotherapy combination therapy.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.