Semaglutide is a hypoglycemic agent used in the treatment of type 2 diabetes mellitus, which is a glucagon-like peptide-1 (GLP-1) receptor agonist that works by mimicking GLP-1 in the human body. It is able to reduce the risk of major adverse cardiovascular events (MACE), including cardiovascular death, nonfatal myocardial infarction, and nonfatal stroke in adult patients with type 2 diabetes with concomitant cardiovascular disease. However, simepaglutide typically requires injectable administration, which may lead to decreased patient compliance, especially in patients with chronic diseases requiring long-term treatment, and frequent injections may compromise the continuity of therapy. Making simepaglutide into microsphere formulations can achieve long-lasting effects, reduce the frequency of administration, and improve patient compliance. In addition, the microsphere formulation can protect the stability of the drug during the transmission process in the body, avoid rapid degradation, and ensure that the active ingredients of the drug can reach the site of action.
Utilizing our microsphere sustained-release platform, we can provide microsphere sustained-release formulations to extend the duration of drug action. In addition, we can ensure the industrialization of microspheres on the ground with a production capacity, and can help customers to do the FDA registration.
 Fig. 1 TEM picture of semaglutide microspheres for injection.
Fig. 1 TEM picture of semaglutide microspheres for injection.
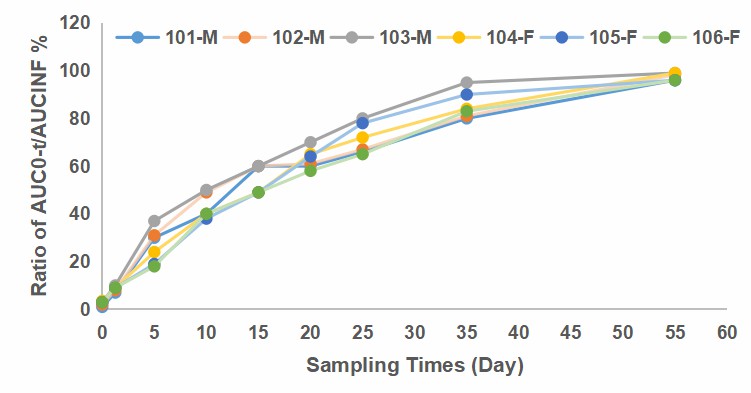 Fig. 2 Time curve of AUCO-/AUCNE percentage in plasma of SD rats after single intramuscular injection.
Fig. 2 Time curve of AUCO-/AUCNE percentage in plasma of SD rats after single intramuscular injection.
Table 1 Comparative experimental results of various formulations of Semaglutide Microspheres in rats.
| Summary of pharmacokinetic parameters | |||||||
|---|---|---|---|---|---|---|---|
| Group | T1/2 (h) | Tmax (h) | Cmax (ng/mL) | AUC(0-t) (h*ng/mL) | AUC(0-∞) (h*ng/mL) | MRT (0-t)(h) | MRT (0-∞)(h) |
| 101-M (Male rats) | 71.8 | 8.00 | 52.5 | 2685 | 3737 | 44.2 | 95.6 |
| 102-M (Male rats) | 70.2 | 8.00 | 37.2 | 1678 | 2242 | 42.1 | 87.2 |
| 103-M (Male rats) | 49.5 | 8.00 | 75.7 | 3854 | 4128 | 51.6 | 64.1 |
| 104-F (Female rats) | 53.6 | 8.00 | 50.8 | 4007 | 4367 | 60.8 | 76.0 |
| 105-F (Female rats) | 52.1 | 8.00 | 71.6 | 3527 | 4032 | 51.0 | 75.1 |
| 106-F (Female rats) | 61.6 | 6.00 | 54.8 | 3140 | 3677 | 58.6 | 87.5 |

Reference
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.