Collagen is a long, thin fiber composed of a triple helix structure, which is formed by various amino acid arrangements. Tendon tissue is the most important tissue structure in mammals. It is composed of various proteins, and collagen is the main protein in connective tissue, with a content of 25% to 35%, which is the most abundant protein in the body. Depending on the degree of biomineralization, collagen tissue may be rigid (bone), flexible (tendon), or have a gradient from rigid to compliant (cartilage). It exists in the cornea, cartilage, bone, blood vessels, intestines, intervertebral discs, and even in the dentin of teeth. In muscle tissue, it is the main component of endomysium. Collagen is one of the main components of muscle tissue. It is secreted by fibroblasts and accounts for 1 to 2%. In strong tendon muscles, the content reaches 6%. There are many types of collagens. According to statistics, 27 types have been discovered and confirmed. Studies have found that type I collagen accounts for more than 90% of the content in animals. Collagen molecules are composed of non-immunogenic repeating unit structures. The two ends of the macromolecule are short amino acid sequences with immune sites, so they have low immunogenicity. This low immunity combined with the unique stable triple helix structure of collagen has enhanced the research value of collagen in medicine. In recent years, collagen materials have been mainly used in tissue materials, implant materials, cosmetic materials, etc., among which the application in medicine is the most extensive. The excellent medical products prepared by collagen have made collagen materials a widely used medical device material. Collagen hemostatic materials, collagen-based bone repair materials, collagen-filled implants, medical oral collagen membranes, collagen dura mater, collagen dura mater, collagen wound dressings, artificial skin, etc. have been developed, showing the wide application of collagen in degradation products, hemostasis, filling, and promotion of tissue repair, as well as its irreplaceable role in many fields.
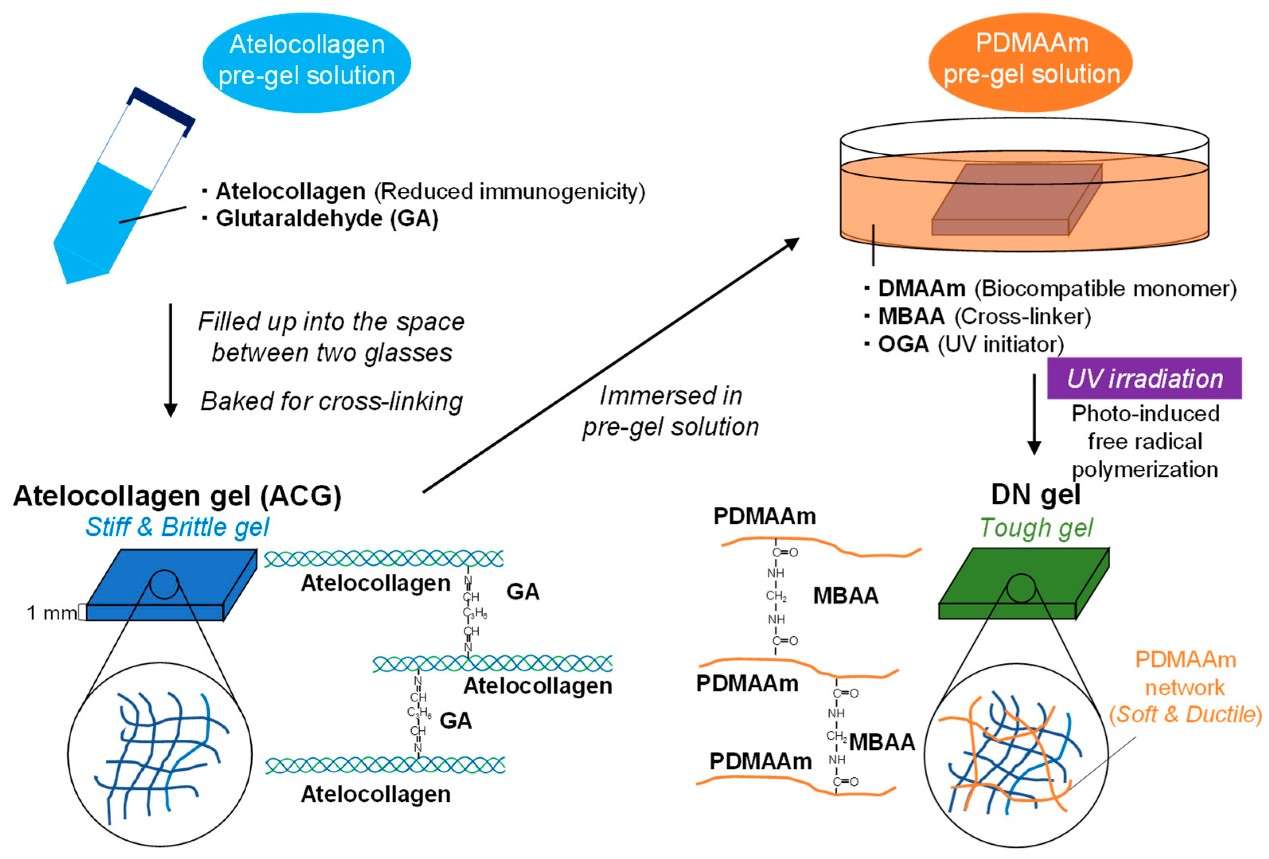 Figure 1. Schematic illustration for preparation of double-network (DN) gel composed of atelocollagen and poly-(N,N-dimethylacrylamide).(Tsuyukubo A, et al.; 2024)
Figure 1. Schematic illustration for preparation of double-network (DN) gel composed of atelocollagen and poly-(N,N-dimethylacrylamide).(Tsuyukubo A, et al.; 2024)
Collagen, like other proteins, is composed of α-amino acids. There are 20 kinds of amino acids. Its amino acid composition has the following characteristics: (1) The main component of protein is glycine, which is almost one-third of the protein content. (2) Collagen molecules have small elasticity and excellent mechanical properties, mainly due to the presence of cyclic amino acids in the molecules, mainly proline and hydroxyproline. This is the main characteristic of collagen molecules. Other proteins contain no or trace amounts of hydroxyproline. (3) The molecular composition of collagen also contains an amino acid-hydroxylysine that is not found in other proteins. This component can promote the adsorption of sugar and promote oxygen glycosylation. (4) Cystine and tryptophan only occupy a small proportion of the collagen molecular components.
Collagen is composed of amino acid sequences, and the main amino acid types are hydroxyproline, lysine, glycine, aspartic acid, etc. Amino acid molecules undergo amide polycondensation reactions to form amide groups. The small molecule chain composed of amino acids is called a peptide chain, and the peptide chain with more than 3 amino acids is called a polypeptide. Collagen molecules are formed by the polymerization reaction of small molecule polypeptide chains to form large molecule chains, and the triple helix structure is formed by the influence of intermolecular hydrogen bonds. The relative molecular weight of collagen molecules is about 3×105 Daltons, white, and easily oxidized and reduced to yellow in the air, so the color of high-quality collagen products on the market is light yellow. Procollagen is the most basic structural unit of collagen. The molecule is composed of three left-handed polypeptide chains entangled with each other, and each polypeptide chain is composed of about 1,000 amino acids. Its amino acid composition can be expressed by (Gly-X-Y)n, where X and Y are two other amino acids. Glycine is arranged at intervals on the collagen molecule chain. This existence of glycine is the key factor for the collagen molecule to form a triple helix structure, which is called the Gly-X-Y repeating region of the collagen molecule peptide chain. X and Y represent two amino acids, proline or hydroxyproline, respectively. They are the key to the formation of stable intermolecular hydrogen bonds in collagen molecules and the key condition for intermolecular forces to maintain the triple helix structure. Since there are many types of amino acids that make up collagen molecules and the ways of combination are also different, the structures of collagen molecules are different, but these structures are all composed of three peptide chains entangled with each other to form a stable triple helix structure, which is consistent in morphology. Each collagen contains at least a triple helix structure region.
The N and C ends of the collagen molecule have an amino acid sequence called telopeptide, which determines the antigenicity of collagen. Collagen with the peptide chain sequence in the telopeptide region removed is called atelocollagen. Atelocollagen is generally obtained by treating calf dermis or type I collagen with pepsin. Due to the low immunogenicity of atelocollagen, it is widely used in the field of biomaterials. It can be prepared into mesh, sponge, powder, and membrane materials for hemostasis, filling, etc. It can also be combined with other biomaterials or mineral materials to prepare composite materials for clinical application. Atelocollagen is the first natural biomaterial with potential application value as a gene carrier. Atelocollagen/DNA complexes can be made into beads, sponges, membranes, microparticles (minipellets), etc. without heating or using any organic solvents. In addition, high-concentration atelocollagen can withstand longer sustained-release time when used as a sustained-release carrier. On the contrary, low concentrations of atelocollagen can be prepared into nano-sized composite particles with a diameter of 100-300nm for systemic application. In addition, the application of atelocollagen for treatment does not change the expression level of related genes, nor does it change the expression level of toxicity-related genes, which shows that it is a good non-toxic and potential candidate gene carrier. Atelocollagen has been successfully used in many in vitro and in vivo gene delivery studies.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.