Drug delivery systems are the technologies of today that allow the delivery of medicines to precise locations in the body. These systems are critical for the treatment of a variety of diseases, since they make drugs bioavailable, have less side effects, and are more effective. It uses drug delivery vehicles to make this possible. They shield the drugs from the environment and carry the drugs to the targeted tissues or cells in specific ways. The human body has natural polysaccharides, such as HA, which are found in connective tissue, epithelial tissues and nervous tissue. HA is biocompatible, biodegradable and non-immunogenic, which is why we frequently use it for delivery. It is a natural drug carrier that can prolong half-life and target more efficiently. Additionally, HA can be more precisely targeted to the tumor cells by interacting with the CD44 receptor on the cell surface and so enhance the drug's localisation at the tumour.
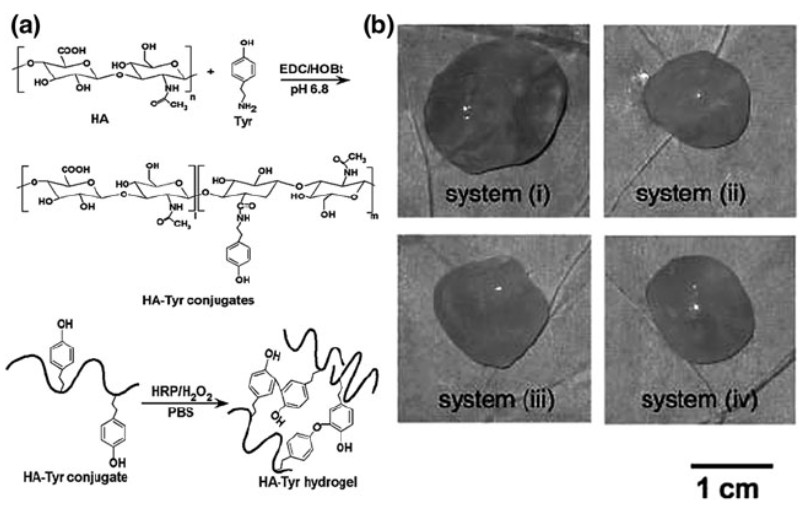 Figure 1. Biodegradable hydrogels consisting of hyaluronic acid–tyramine conjugates. (I. M. El-Sherbiny, et al.; 2018)
Figure 1. Biodegradable hydrogels consisting of hyaluronic acid–tyramine conjugates. (I. M. El-Sherbiny, et al.; 2018)
HA has a lot of advantages, but it is not stable and easily damaged by strong acid, strong alkali, heat and enzymes, so it is not used in some drug delivery. Overcoming these restrictions, chemists have chemically altered HA by esterification, cross-linking and grafting to give it mechanical strength and stability. The use-case for these modified HA derivatives in the form of nanoparticles, hydrogels and microneedles has been expanded. As medical technology has evolved in the last few years, there has been an exponential rise in the demand for new carriers for drug delivery. A new drug delivery vehicle, hyaluronic acid tyrosine (HA-Tyrosine) is increasingly popular for its chemical and biological features. HA-Tyrosine has the biocompatibility of hyaluronic acid with the biological activity of tyrosine and offers many benefits in the drug delivery chain such as targeting and drug efficacy. This article is about using hyaluronic acid tyrosine as a drug delivery agent. Using basic facts about HA and its use for drug delivery, this post will explain the popularity of newer systems like hyaluronic acid tyrosine and what the future of HA-based drug delivery could be.
Hyaluronic acid is a mixture of different disaccharides such as β-1,4-D-glucuronic acid and β-1,3-N-acetyl-D-glucosamine bonded to one another by glycosidic chains in a linear polysaccharide chain. This makes HA extremely high molecular weight, hydrophilic and viscoelastic. HA is molecularly weighted in many different ways, from tens of thousands of low molecular weights to millions of Daltons of high molecular weight, and HA with different molecular weights has various biological activities. HA has many functions in the extracellular matrix – hydrating, lubricating, regulating cell signals, healing wounds and repairing tissue.
Hyaluronic acid tyramide (HA-T) is an alkylated HA derivative, made by tyramide modification. Hyaluronic acid tyramide (HA-T) is a derivative that comes about by putting tyramide groups on the molecule of hyaluronic acid. It's a modification that not only makes HA more chemically reactive but also confers novel functional characteristics like biocompatibility and targeting. When HA-T is produced, it is normally chemically reacted with HA to make a stable covalent bond.
HA-T delivers targeted drugs by interacting with receptors on the cell surface, including CD44. CD44 receptors are highly over-expressed in a wide range of tumor cells and HA-T can exploit this feature to precisely deliver drugs to the tumor cell where they'll have the best therapeutic effect and minimise toxic effects in normal tissue. Furthermore, the cross-linked network architecture of HA-T can modulate the rate of drug release, make the drug be released gradually in the body, and extend duration of action. HA-T is more stable and specific than natural HA. Natural HA is well-biocompatible and biodegradable but low in stability and easily broken down by enzymes. By changing it with tyramine, HA-T is not only stable but also enhanced in terms of drug encapsulation and release. Moreover, HA-T mechanical strength and gelation rate can be adjusted to a high or low concentration depending on the needs of the drug delivery.
More biocompatibility: HA-T is immunogenic, as it has been chemically modified, so it's biocompatible in the body.
Reduce immunogenicity: Tyramine modification makes it less likely for HA-T to bind to antibodies in the body and hence inhibits the immune response.
Unique targeting: By specific binding to the CD44 receptor, HA-T targets the drug accurately to the tumour cells for greater therapeutic efficacy and decreased side effects.
While natural HA is biocompatible and biodegradable, it is less stable and easily digested by enzymes, which makes it not a useful form of delivery for pharmaceutical purposes. HA-T, on the other hand, is greatly improved in stability by tyramine modification, and both the release rate and encapsulation rate can be optimized by changing the amount of tyramine.
Drug Delivery Applications of Hyaluronic Acid Tyramide
There are numerous uses for HA-T as a delivery agent in drug delivery including targeted delivery, hydrophobic drug encapsulation, controlled and sustained release delivery, and gene delivery.
Targeted drug delivery systems make use of HA because it binds strongly to CD44 receptors on the surface of tumour cells. The CD44 receptors are over-reported in a range of tumour cells, which means that HA-based drug delivery systems can target the tumor tissue with the right drug, via the receptor network, and without damaging adjacent healthy tissue. Further, HA can also be stored in the tumor microenvironment via the EPR effect (proportional permeability and retention effect), increasing drug targeting and therapy efficacy.
HA is both biocompatible and chemically stable and it can be used to package hydrophobic drugs either physically or chemically. For instance, HA micromicelles (PMs) can contain small molecule anticancer agents in their hydrophobic nuclei and release drugs under controlled conditions (PH, temperature, enzymes, etc.). Further, HA can also be fused to hydrophobic polymers to create complexes that make hydrophobic drugs solubilised and stable.
HA-based delivery systems can also be controlled to release drugs by modulating molecular geometry and cross-linking level. pH-sensitive HA-PLGA micelles can release drug when they encounter acidic conditions and extend the time of drug action and efficacy. Furthermore, HA can also be incorporated with other reactive molecules like redox-sensitive polymers for more specific drug release control.
Hyaluronic acid has potential for gene delivery too. HA can be used as a gene carrier to introduce siRNA, mRNA or plasmid DNA into cells. The delivery of siRNA could be boosted by HA-tagged liposomes, for instance, which shield siRNA from enzyme degradation in serum. HA can also be mixed with other cationic polymers to make complexes that enhance the specificity and efficiency of gene delivery.
Hyaluronic acid tyramide has large application potential in drug delivery, particularly targeted drug delivery, hydrophobic drug encapsulation, controlled and sustained release, and gene delivery. It's a useful tool for cancer treatment and tissue repair.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.