Tumour treatment has been a rapid-moving industry in the past couple of years and one of the tumour cures are tumor vaccines. They are made from tumor-associated antigens (TAAs), tumor peptides, or tumour cell lysis products, etc. and enhance T-cell proliferation, activation and function by regulating the body's innate or acquired immune system, stimulating cytokine release, enhances the tumor microenvironment, enhances T cells to detect and destroy tumor cells, reduces the proliferation of tumor cells, and prevents and treats tumors. One of the most relevant tumor vaccines are tumor exosome vaccines (EV). And as the research on tumor exosome vaccines advances, evidence of its distinct benefits keeps accumulating. In this paper, we look at exosome vaccines for tumour vaccines. Structure/function of the Exosome Exosomes (EX) are cell-derived nanoscale (30-140 nm diameter) extracellular vesicles, coated with a lipid bilayer. They are found in almost all biological fluids: blood, urine, milk, respiratory secretions, saliva, cell culture medium. EX is packed with membrane proteins and soluble factors that are important to cell function: lipids, proteins, nucleic acids. Evidence showed that EX acts in multiple critical functions of normal physiology and pathophysiology, and is able to relay messages from host cells to target cells over great distances, such as antigen presentation, infection immune monitoring, intercellular signalling, different proteins and RNA releases, and infectious agent transfer. Because EX is suspended in biological fluids, its source-dependent composition can be very reflective of a range of physiological and pathological states, and it is a perfect non-invasive or minimally invasive tool for diagnosis and treatment efficacy tracking.
Cancer can be controlled by tumor cell EX. TEX are tumescent exosomes (as in tumor-derived or tumor-specific antigen) that are immunogenic. They elicit anti-tumor immunity by feeding tumor antigens to APCs, like dendritic cells (DCs). Meanwhile, tumor-derived exosomes could also aid epithelial-mesenchymal switchover. Hypoxia-inducible factor 1 (HIF1), -catenin, caveolin-1 and transforming growth factor (TGF-) all make exosomes capable of the invasion and migration of tumour cells. Exosomes of tumor cells, then, also remodel the pre-metastatic microenvironment and matrix. EX is a non-cellular antigen that can expressing the molecules of its progenitor cell, and is exactly like the progenitor cell. It is a natural drug delivery system that, as an endogenous natural drug delivery system, has distinct advantages: (1) Exosomes are high in lipids/proteins that can penetrate the biological membrane and therefore facilitate effective drug delivery; (2) nanoparticles of exosomes can gather antigens and be absorbed by DC cells; (3) exosomes are intrinsically tissue compatible, low in biological toxicity and better concentrated for focusing antigens. That is exactly why exosomes are so special, making cell-based exosomes a fresh new delivery vehicle for vaccine research.
Exosomes as tumor vaccines were always an interesting possibility in the context of tumor immunology. As per their source cell, they are categorized into the following categories: (1) exosomes from tumour cells; (2) exosomes from immune cells like dendritic cells; (3) exosomes from stem cells; and (4) exosomes from other sources. When developing tumor vaccines using exosomes, as tumor antigens, this approach is more advantageous than tumour cells or tumor cell lysates.
The exosomes of tumour cells are full of tumour antigens and tumor antigens, as well as some proteins, like major histocompatibility complex type I and II (MHC-I and MHC-II), phosphatidylserine, milk fat globulin 8, liposome-associated membrane protein 1, annexin II, CD9, CD81, CD54, CD63, etc. These protein clusters drive EX recognition and recognition by ligand-like proteins on DC cells, recruit a large assortment of tumor antigens to DC cells, activate tumour-specific cytotoxic T lymphocytes (CTL), and inducing potent anti-tumor immunity. Meanwhile, to neutralise exosomes' immunosuppressive capacity in themselves, tumour cells are genetically engineered to produce exosomes with elevated antigen or activation factors expression, which might in turn be better at activating immune cells, augmenting the microenvironment of the tumour, activating anti-tumor cell immune responses, and inhibiting tumor cell growth, recurrence and metastasis.
Dendritic-cell Exosome Vaccines from Dendritic Cells
DC cell exosomes (DEX) have all the necessary immunostimulatory components of DC cells – MHC-I/MHC-II complexes, high concentration of co-stimulatory molecules, etc. – to replace the cell in order to overcome the disadvantages of DC cell therapy (high cost, time required, difficulty in preserving living cells). In the first place, the fixed membrane of the exosomes lends Exo a better ability to be manufactured and stored. Second, being the smallest antigen presenting unit, Exo does not get impacted by the environment and can continue to function and look the way it does. At last, cell-free vaccines avoid live DC-based risk of viral infection and in vivo replication.
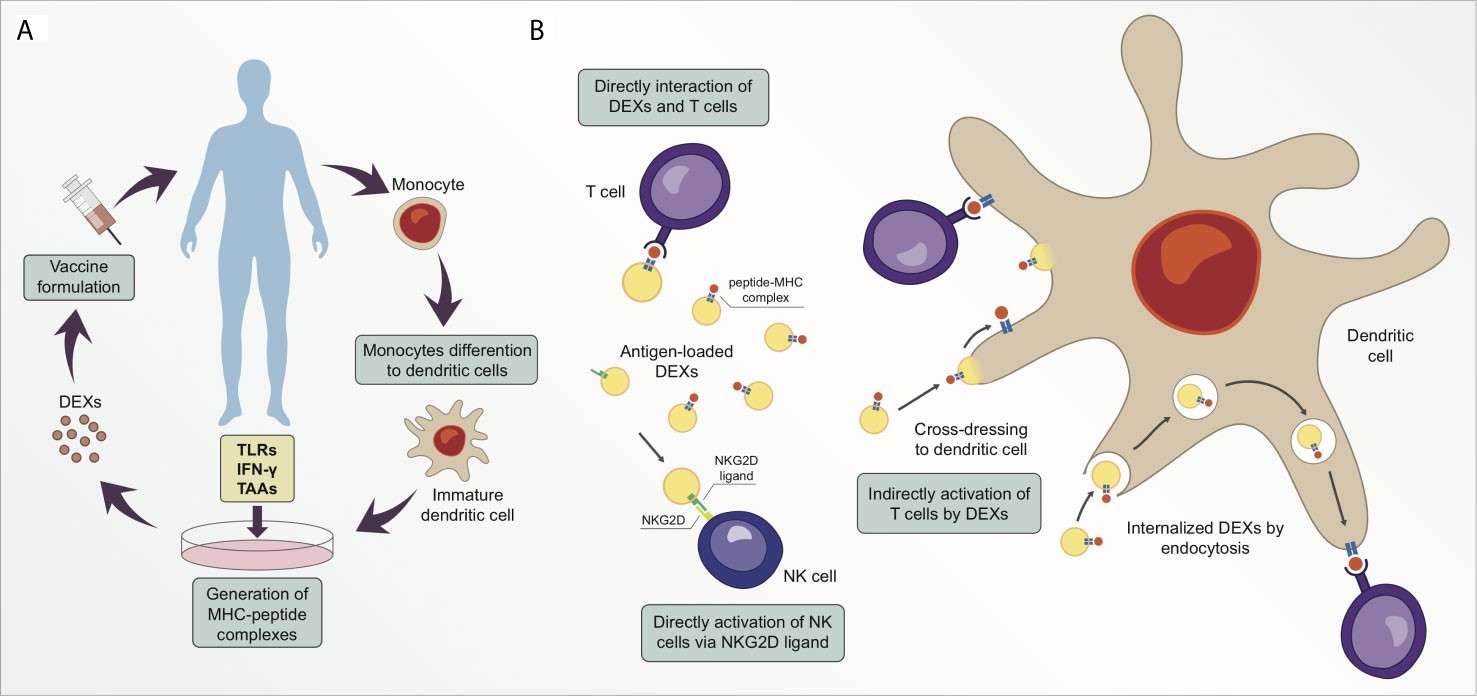 Figure 1. Exosomes derived from dendritic cells (DEXs) are potential targets for cancer therapeutic strategy. (Santos P, et al.; 2021)
Figure 1. Exosomes derived from dendritic cells (DEXs) are potential targets for cancer therapeutic strategy. (Santos P, et al.; 2021)
We've discovered that exosomes released by mouse DC cells carrying the AFP gene can enhance the tumour microenvironment in mice with ectopic and orthotopic liver cancer, particularly in orthotopic liver cancer mice inducible by diethylnitrosamine (DENA). The tumor immune microenvironment is transformed from an immunosuppressive to an immunostimulatory milieu. This raises the count of CD8+ T cells in the tumour microenvironment, IFN- and IL-2 levels, the number of CD25+ Foxp3+ regulatory T (Treg) cells, IL-10 and TGF- levels, all of which can activate robust antigen-specific immune responses and act as tumour suppressors.
Macrophage-derived Exosome Vaccines
Exosomes are packed with parental-cell RNA and proteins, so exosomes released by inflammatory immune cells can boost vaccine immunity. Exosomes released by enflamed macrophages contribute to both innate immunity and adaptive immunity. As macrophages differentiate in light of a different stimulus from the environment, they are usually segmented into M1 and M2 cell populations. Traditionally activated macrophages secrete inflammatory chemicals to kill pathogenic microbes and drive Th1 immune activity. Not just cytokines and chemokines, but other bioactive molecules involved in local microenvironmental immune regulation are released by macrophages, including exosomes. M1 macrophage exosomes release inflammatory signals and create a local immune stimulatory microenvironment, so they could serve as immune adjuvants for vaccinations.
The same antigens are shared between ESCs and tumour cells ('non-self' antigens, as these antigens don't normally appear or only show minimal activity in normal adult tissues). If ESC exosomes are similar to tumour cells because they share antigenic homology with cancer cells, ESC exosomes can be activated by anti-tumour immunity and tumour cell death and could function as tumor vaccines.
Human anogenital tumours are mainly caused by papillomavirus. Current related vaccine treatment is a good tool to delay tumour formation. For the HPV-16 virus vaccine, they used DNA vectors carrying Nefmut (an exosome anchoring protein)/E7 fusion protein to inoculate mice with intramuscular vaccines, which combined with nanovesicles spontaneously generated by all cells, including muscle cells at the injection site, to create endogenous engineered exosomes that generated effective and unbounded cytotoxic T lymphocyte (CTL) immune responses against HPV-16-E7 tumor antigens and thus had anti-tumour therapeutic properties. The interaction of exosomes from different cells in order to act on their respective roles in the anti-tumor immune system is also one of the research areas.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.