Introduction
Liposomes are artificial membrane structures made of phospholipid bilayers that could be filled with hydrophilic and hydrophobic drug molecules. This can be done in various ways like by thin film dispersion, reverse phase evaporation, injection, etc. Liposomes provide a great way to deliver drugs because they have an interesting structural makeup (hollow spherical structure, particle size control and surface functionalization). What makes liposome technology so unique is that it allows drugs to become more stable and bioavailable, and less toxic to healthy cells. Furthermore, liposomes can be targeted via surface modification (i.e., targeting of cells to particular cell populations through antibody or ligand binding). The medical application potential of liposomes is huge in cancer therapy, gene therapy and vaccines. Liposomes could be put to work in capsules to wrap anticancer drugs, target the damage to healthy tissue, and maximise efficacy. They are also applied to gene therapy and vaccination to enhance the delivery of nucleic acid molecules to their intended cells through liposome protection from enzyme degraded in the body. The liposomes can also be utilized to deliver diagnostic reagents for early detection and treatment tracking of diseases, like tumors.
What is Liposomal Delivery?
Liposomes are tiny vesicle particles made of phospholipids and other molecules of lipid. They are double-layer membranes that can be packed with hydrophilic and hydrophobic drugs. It's no longer just liposomes as a perfect delivery platform for drugs because of their specific surface area, biocompatibility and controllable drug loading capacity – it's the physicochemical advantages that make them such a desirable drug carrier.
Having been discovered in 1965, liposome technology has had a long road from laboratory to clinic. Researchers first learned that liposomes mimic cell membranes and are used to investigate the physiology of cell membranes. People had been talking about liposomes as drug delivery systems in the 1970s, and had theories and approaches. Lipososomes were also refined and improved in the 1980s, in particular its stability in biological fluids like blood. Then, liposome technology gradually took off from the lab and entered the clinic as a common drug delivery method.
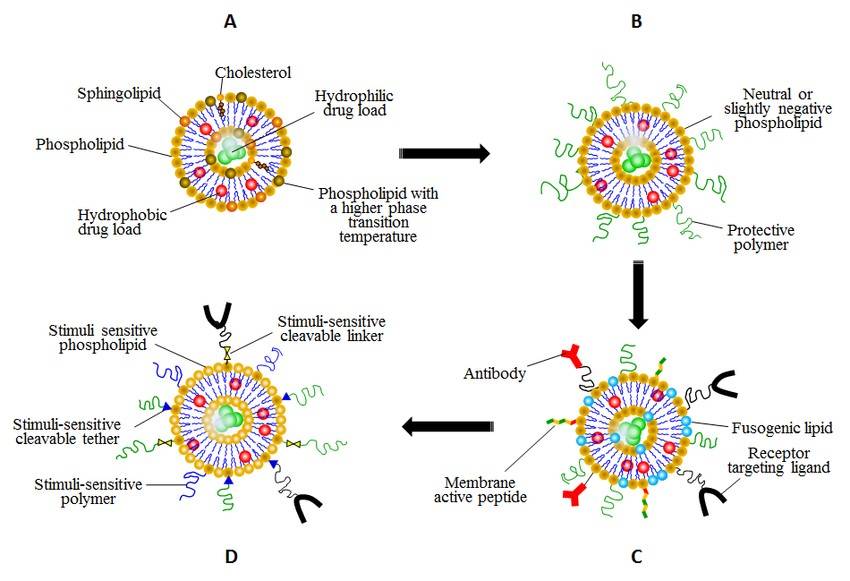 Figure 1. Evolutionary steps of liposomal drug delivery systems in order of their complexity. (Sergey Kazakov, et al.; 2016)
Figure 1. Evolutionary steps of liposomal drug delivery systems in order of their complexity. (Sergey Kazakov, et al.; 2016)
Comparison Between Liposome Delivery and Conventional Drug Delivery
The advantages and features of liposome-mediated delivery compared to the existing forms of drug delivery:
1. Stabilization of drugs: Liposomes can prevent drugs from enzymatic breakdown, prolong drug half-life, and enhance the drug's bioavailability.
2. Targeting: By altering their surface liposomes are capable of targeted drug delivery (eg, precisely binding to the right cells or tissues with antibodies or ligands).
3. Decrease side effects: Liposomes can prevent drug from collecting in the body outside of its intended targets and having negative side effects in healthy tissues.
4. Versatility: The technology of today's liposomes has envisioned different liposomes, like long-circulating liposomes, environmental sensitive liposomes, actively targeted liposomes, etc. These novel liposomes have been demonstrated to be highly potential for anti-tumor, anti-infection, vaccine, etc.
The Structure and Composition of Liposomes
Liposome Structure
The basic liposome is composed of a bilayer membrane consisting of hydrophilic head and hydrophobic tail molecules of phospholipids. Phospholipid molecules self-stack an elastic water-water bilayer membrane and hold water-soluble molecules within. We can distil liposomes into unilamellar and multilamellar liposomes. Monolamellar liposomes are composed of a membrane of phospholipid bilayers; multilamellar liposomes are composed of stacked phospholipid bilayers.
Liposome Composition
Phospholipids and cholesterol make up the majority of liposomes. They're the building blocks of liposomes, the double shell. Phospholipid molecules are hydrophilic so create durable bilayer membranes in water. Cholesterol makes the liposome more stable by making the membrane more pliable and by decreasing the crosstalk between phospholipid molecules. At high levels, mol-rate ratio of cholesterol and phospholipid is 1 or 2.
Liposome Sizes and Their Relevance
A liposome ranging from nanometers to micrometres in size isn't the only size you can imagine. Liposomes are of the following types according to particle size and layer number:
Unilamellar liposomes (SUV): A membrane-like particle, 20–100 nanometres in diameter, that's commonly used to coat hydrophilic active materials.
Medium multilamellar liposomes (MUV): Between 100 and 500 nanometres long, which is better than unilamellar liposomes, with more hydrophilic material.
Mega-Multilamellar Liposomes (LUV): 500-1000 nanometres, larger size, for packaging hydrophobic drugs.
Multivesicular liposomes (MPLV): They are from 1 to 5 micrometers, consisting of many small vesicles, which are useful for encapsulating large molecular molecules drugs.
Liposomes of different sizes have different drug-delivery applications. Small unilamellar liposomes (SUV) are useful for encapsulating hydrophilic small molecules; large multivesicular liposomes (MPLV) are useful for encapsulating large molecular bioactive molecules, like proteins and antibodies.
Benefits of Liposomal Delivery
Liposomes can make drugs much more bioavailable. Liposome-encapsulated medications, for instance, don't get broken down in the gut, maximizing the absorption rate of water and fat-soluble drugs. Furthermore, liposomes can even improve drug efficacy by extending the time for drugs to travel in the body.
- Targeted Delivery to Specific Cells or Tissues
Liposomes are very selective and could be delivered with surface modifications (antibodies, ligands, etc.) Liposomes that attach to receptors on the outside of cancer cells, for example, can drive drugs into the tumour without toxicating surrounding tissue.
- Eliminate Side Effects and Make It Safer
Liposome-rich drugs are often less harmful to normal tissue and therefore safer to use. Liposome-encapsulated drugs, for example, don't clog livers and are thus less damaging to the rest of the body. Also, liposomes can reduce how many times the drug is delivered by controlling the dose of release, which reduces side effects as well.
Because of its physicochemical novelty and adaptability, liposome delivery has proven successful in drug bioavailability, targeted delivery, adverse effect mitigation, and medical applications.
Applications of Liposomal Delivery
As a drug delivery system, liposomes are used in multiple fields. Here are some of liposome's usages in other areas:
Drug Delivery for All Types of Diseases (Cancer, Infection, etc.)
The potential for liposomes has been huge in cancer therapy, as they render medications more stable and permeable and without side effects. Liposomes could also carry chemotherapy drugs (cisplatin, doxorubicin, paclitaxel, and the like) for greater efficacy and less toxicity, for instance. Other uses for liposomes include delivering drugs to tumor cells, including mixing antibodies or transmembrane peptide modifications to target cancer cells exactly.
Lipososomes in infectious diseases can be injected with vaccines and used in immunotherapy such as to transfer vaccine ingredients to cells through liposome technology for increased immunity.
To Take Supplements Nutrition (Vitamins, Minerals)
The reason liposomes are used in nutrition supplements is simply to enhance vitamin and mineral bioavailability. Liposome-encapsulated vitamins C and D, for example, could be more readily taken up into the body and hence more nutritious.
Liposomes have been known to help minerals go better down and the minerals (iron and magnesium, for example) are able to be used more efficiently in the body.
New Applications in Cosmetics and Other Industries
The liposomes in cosmetics stabilize actives and make them less permeabile to the skin. Liposomes, for example, might be used to cap antioxidants and sunscreens so that they could be put to the best possible use in cosmetics.
Then there are the food and beverage applications of liposomes that serve to defend enzyme bioactive molecules, extend their shelf life in food, and increase their absorption through the gut.
Future Trends and Innovations in Liposome Technology
The future of liposome technology will be about the following:
1. Multifunctionality and targeting: Target and stabilize the liposomes even more with surface engineering and functional design. For exmple, make new liposome types like long-circulating liposomes, environmentally sensitive liposomes, and targeted liposomes.
2. Drug loading and controlled release technology: Enable liposomes to be loaded with more drugs and can be capsulated, or create controlled release technology for time- or place-specific release.
3. Production and process optimization at scale: Scale up the production of liposome preparations, decrease production cost and increase production efficiencies to fulfill large commercial demands.
4. Extension of new application fields: The application areas of Liposomes are now expanding to various diseases including neuroprotective drugs, gene therapy, and immunotherapies.
Challenges and Considerations in Liposomal Delivery
Stability and Shelf-Life Issues
1. Stability: Liposomes aggregate and fuse, so they are less stable. This needs to be kept in check, by controlling the particle size distribution, surface charge, temperature and pH at preparation.
2. Stability: Liposomes are made of phospholipids, which are easily oxidized and hydrolyzed, in particular unsaturated double bonds are easily oxidized, thus liposome stability is affected. Furthermore, liposomes can leak or decay during storage, and the released drug could not be released at the right time.
3. Stability biologically: There is also stability of liposomes in vivo including recognition and clearance by the immune system. Surface modification like PEGylation can extend liposomes' circulatory lifetime and stabilise them.
Manufacturing and Scalability Challenges
1. Complexity of manufacture: Liposome production is typically a multistep process, such as dissolution, mixing, emulsification and drying, which adds to production cost and time.
2. Scalability problems: The lab scale liposome manufacturing is hard to immediately scale up to large production which hinders the introduction and application of novel liposome systems. Technologies like microfluidics and static mixers are also being explored to increase the production efficiency and quality.
3. Hardware and technical restrictions: In order to manufacture, we need the equipment and technical assistance like supercritical CO2-aided technology, spray drying etc. It's expensive to deploy such technologies.
Compliance and Approval Procedures (Regulatory)
1. Complete sterility, batch-to-batch consistency: Liposome drugs require pharmaceutical-quality control measures like sterility and batch-to-batch consistency. Every production process has to be documented in detail for regulatory purposes.
2. Issues of Immunogenicity: Liposomes could induce immune response and interfere with drug safety and effectiveness. Therefore, immunogenicity studies must be conducted in the early stages of development and immunological suppression measures must be implemented.
3. Adoption: It takes some time for liposome drugs to get approved and there is a series of clinical trials that are needed to validate their safety and efficacy. Meanwhile, regulatory conditions can also influence approval timelines and outcomes.
References
- Sergey Kazakov, et al.; Lipobeads as Future Drug Delivery System. Nanotechnology in Drug Delivery. One Central Press (OCP). 2016, pp.85-129.
- Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013, 65(1):36-48.


 Figure 1. Evolutionary steps of liposomal drug delivery systems in order of their complexity. (Sergey Kazakov, et al.; 2016)
Figure 1. Evolutionary steps of liposomal drug delivery systems in order of their complexity. (Sergey Kazakov, et al.; 2016)