Gene therapy is a hot target as a potential cure. Not only can it cure genetic disorders, but also other acquired disorders such as cancer, cystic fibrosis, AIDS, etc. Different antisense oligonucleotides enter the cell primarily via endocytosis but the negative-charged main chain significantly restricts their penetration into cells and transfection of them. Nowadays, the non-viral vectors delivery of nucleic acid molecules is a common gene therapy approach. These include cationic liposomes, which have been popular in clinical diagnosis and gene therapy because they are simple to prepare, reproducible and biodegradable.
Cationic liposomes mostly made of cationic lipids. Not only can lipids compose liposomes by themselves, but lipids can compose liposomes with other secondary lipids. Most commonly used cationic lipids are made up of three molecules: hydrophilic head groups, hydrophobic tail groups and the connecting bonds between them.
These positively charged hydrophilic head groups carry RNA between liposomes and nucleotides, membranes or other cell structures, and aid in gene delivery. Head groups can be classified as: quaternary ammonium, amine, amino acid or polypeptide, guanidine, heterocyclic head groups and some special head groups. The other recent trend in the study of cationic liposomes is to integrate some groups like mannose, galactose or fluorescent into the head group domain of cationic lipids and increase the rate of transfection in vitro and in vivo. Other cationic lipids (cardiolipin, phosphoramide derivatives, etc.) with similar molecular configurations as some living species — are less cytotoxic and more effective in transfection, so the outlook is promising for cationic liposomes to be used for human gene therapy.
The hydrophobic tail group has two major groups: cholesterol ring derivatives and fatty acyl chain derivatives. Auxiliary lipids are neutral lipids, mainly DOPE (1,2-dioleylglycerol-3-phosphatidylethanolamine), PC (1,2-diacylglycerol-phosphatidylcholine), DOPC (1,2-dioleylglycerol-3-phosphatidylcholine), DPPC (1,2-dipalmitylglycerol-3-phosphatidylcholine), Chol (cholesterol), etc. They can stabilize the lipid bilayer membrane and make cationic lipids less toxic.
The linking relationship between head and tail group controls both the chemical stability and biodegradability of cationic lipids, and also influences their transfection efficiency and cytotoxicity. Dependent on the configuration of the linking bond, they can be classified into different types — ethers, esters, amides, disulfides, ureas, acylhydrazones, phosphates and other special cases. Linkers of this kind, ethers were the original. The more cationic liposomes with ether linkers are generally more effective for transfection but, because ether linkers are chemically immobilised and not biodegradable, they are also more toxic. Unlike ether linkers, biodegradable esters added to cationic lipids can contribute to intracellular oxidative metabolism or dissolving lipids so that cells don't languish with non-natural lipids and the deleterious effects that can follow. The researchers should also be fully considered: what is the length, type and position of the linker are of enormous importance to the rational construction of novel cationic lipids with high transfection efficiency and low cytotoxicity.
Caitonic liposomes as gene carriers cling to negatively charged target gene sequences via electrostatic attraction. Transfection comes mostly via membrane fusion or endocytosis. The lipid complex releases the gene in the cytoplasm or into the nucleus and transcription and translation are complete within the cell. As well as the shape of the cationic lipid and the kind of biological response that needs to be characterized, the transfection capacity of cationic liposomes can be also influenced by the gene that's being delivered. For instance, plasmid DNA (pDNA) is more efficiently delivered to cells than linear DNA since pDNA has a less effective negative charge than linear DNA, so we don't need as many cationic lipids.
Because pDNA, mRNA, siRNA, microRNA and other gene-therapy antisense oligonucleotides mostly enter cells through endocytosis but because their negatively charged main chains greatly inhibit cell contact and targeted cell transfection, cationic liposomes can carry them to the nucleus or cytosol to be used for therapeutic purposes.
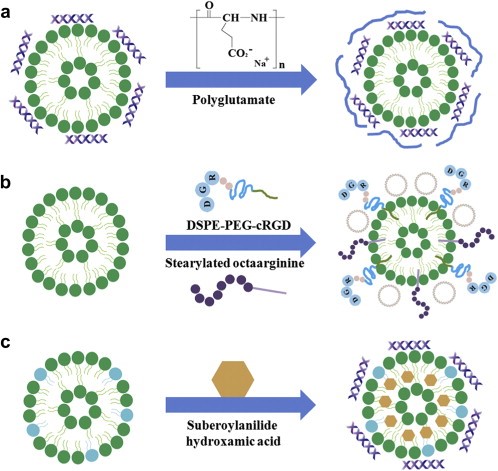 Figure 1. Strategies for cationic liposome-based nucleic acid delivery. (Gayong Shim, et al.; 2013)
Figure 1. Strategies for cationic liposome-based nucleic acid delivery. (Gayong Shim, et al.; 2013)
pDNA is also a hot product as a biomedicine. It can be used as a gene therapy vehicle because it is safe and stable, has high potency for many different exogenous genes, is simple to manufacture, and doesn't directly interfere with the human immune system. But its transport isn't without obstacles: it needs to have good load-ability and not get aggregated by the pDNA's high negative charge and heavy molecular weight. Especially since, unlike mRNA, pDNA must get to the nucleus rather than just the cytoplasm to translate proteins. We commonly choose biocompatible amino acids as lipid components for cationic liposomes, and cationic liposomes enriched with DOPE promote in vivo pDNA transport.
Messenger RNA (mRNA) is a natural product of genes and, in cells, its job is mainly to carry genes and to act as an immediate template for the construction of proteins. Clinical investigations of mRNA today are confined to vaccines, protein-replacement therapy and gene therapies. But the use of mRNA is still limited as it remains to be improved in its way of delivery.
SiRNA is a small interfering RNA that can block particular genes that cause virus infection and cancer, and could be therapeutic. SiRNA is lighter (it has only 20–30 base pairs) than pDNA and it doesn't have to travel to the nucleus to do its job. And siRNA is more specific than microRNA. Nevertheless, siRNA has many hurdles still to overcome in application such as off-target effects, administration difficulties, immune response and cytotoxicity. As it is, different siRNA drug formulated cationic liposomes are currently in clinical trials; most common are stable nucleic acid particles (SNALPs) consisting of cationic lipids, fusogenic lipids, cholesterol and polyethylene glycol (PEG). Positively charged cationic liposomes, although ideal for transmembrane transport, also dock with blood proteins. So, when injected into serum, cationic liposomes are rapidly elutriated from the circulation system and siRNA is released. Additionally, cationic lipids are cytotoxic (ie they degrade cell membranes and create reactive oxygen species). To increase the transfection efficiency of siRNA and decrease the cytotoxicity, as well as choosing the carriers' components, transferring materials to more stable cationic liposomes is also popular. Most commonly used other substances include polyethylene glycol (PEG), hyaluronic acid (HA), hydrophilic peptides, etc.
In animals and plants, microRNA (miR) is most often devoted to regulation of post-transcriptional gene expression. Moreover, delivery of miR through synthetic cationic liposomes could suppress mRNA translation and other key gene functions, so it's therapeutically active.
Given the effect of cationic liposomes on physicochemical structure and human body physiology, there are still hurdles to gene therapy in clinical practice.
Our body's internal landscape isn't that straightforward: opsonins at the endocellular barrier, the clearing of the reticuloendothelial system, poor penetration of tumors. This barrier is the intracellular wall composed of the endosomal/lysosomal transport system and the relatively weak intranuclear diffusion that prevents cationic liposomes containing exogenous genes from getting to the site. Conveniently, cationic liposomes can be modified by changing the lipid composition and surface modifications like PEG modifiers to make them effective carriers of nucleic acids and therapeutic agents.
And where cationic liposomes end up in the body not only depends on the physiological context, but also on their physicochemical properties (which are strongly influenced by the lipid composition). For example, the molecular shape of cationic lipids and the melting of neutral auxiliary lipids are critical for maintaining efficiency in transfection. Meanwhile, the effectiveness of transfection depends on the particle size and surface charge of cationic liposomes (both influenced by the composition of the lipids).
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.