Bangham in Cambridge had discovered and purified liposomes first in 1965. They're a microsphere with a special ability to house water-soluble or fat-soluble drug in lipid bilayers and hollow spaces. Simultaneously, as a drug carrier, liposomes can make encapsulated drugs more solubilized and stable, as well as more targeted, which decreases peripheral adverse reactions of drugs. Because tumors are so invasive and destructive, anti-tumour research has always been a hot topic and challenge. Liposomes are also non-immunogenic and can be used to make use of the EPR (high permeability and retention effect) on the surface of the tumor, and interfere with tumor cells by changing their surface.
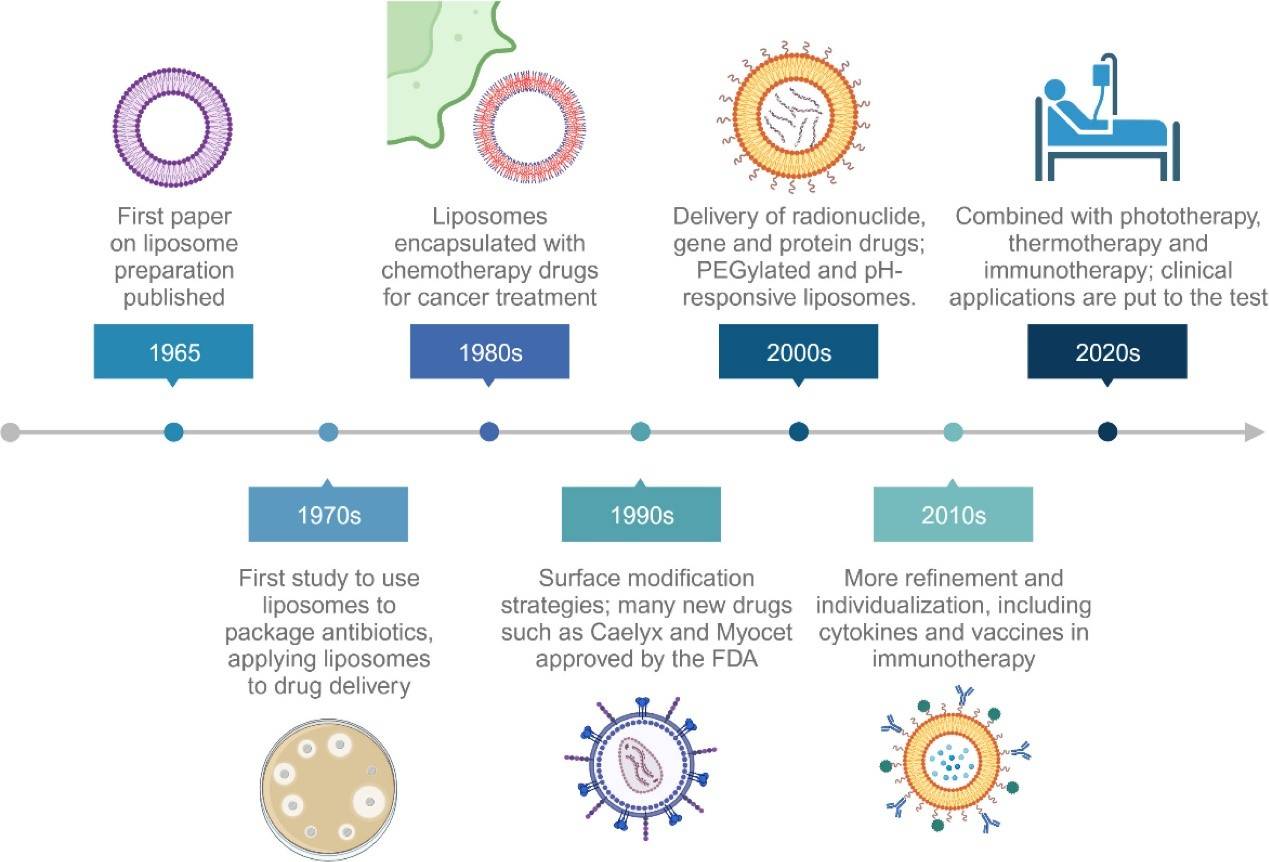 Figure 1. A historical timeline of major developments in cancer liposome medicine. (Chen J, et al.; 2024)
Figure 1. A historical timeline of major developments in cancer liposome medicine. (Chen J, et al.; 2024)
Liposomes are microvesicles with one or more aqueous chambers made up of lipid bilayers, and typically made up of phospholipids and cholesterol in some ratio. There is a bilayer structure made of phospholipids, and there's cholesterol that's also responsible for securing and maintaining the bilayer. The two complement one another and stabilize liposomes. The core-shell design of liposomes is distinctive, it's biocompatible and biodegradable, it's able to insulate sensitive drugs against the environment (light, temperature, pH, etc.), and make drugs more effective by regulating drug release. Liposomes can be classified into unilamellar liposomes and multilamellar liposomes depending on the number of lipid bilayers. Small unilamellar liposomes (particle size 20-100nm, good membrane stability, low encapsulation rate, low drug loading; large unilamellar liposomes are single-layer large vesicles (high water-soluble drug encapsulation rate, particle size 100-1000nm, high drug loading, poor membrane stability); giant unilamellar liposomes (particle size >1000nm, near-zero membrane tension). Multilamelliposomes are multilamellar liposomes. Multilamellar liposomes can have particle size between 8-12 m, multiple lipid bilayers (mostly greater than 5 layers), high stability and high encapsulation rate, and can be designed and produced for the design of long-term release preparations.
Passive Targeting Liposomes
Passive targeting means that liposomes can cause spontaneous retention based on the difference between the high permeability of tumor tissue and the microvascular structure of normal tissue, forming a natural tendency of enrichment. Traditional passive targeting liposomes are easily affected by substances such as laminin and complement proteins, and are thus recognized and taken up by macrophages, resulting in the rapid clearance of their contained drugs in the body. Therefore, long-circulating liposomes obtained by surface modification with polyethylene glycol (PEG), phosphatidylinositol, etc. are a major progress in the development of passively targeted liposomes. The liposome's metabolism rate in the body is significantly reduced, the systemic circulation time of the drug is significantly prolonged, and the EPR effect is increased, making it easier for the drug to accumulate at the lesion site, reducing immune organ toxicity and adverse reactions. In the preparation process of long-circulating liposomes, PEG is the most widely used. As an extension of the hydrophilic end of long-circulating liposomes, PEG can increase steric hindrance, making it impossible for plasma opsonic proteins to adhere and aggregate with liposomes, thus protecting liposomes from being recognized in the blood, thereby reducing the risk of reticuloendothelial system intake.
Immunoliposomes – liposomes that introduce monoclonal antibodies or functional segments into the liposome surface, and exploit the antibody's selective recognition power to make the drug richer in the target tissue, minimising toxic side effects of the drug and increasing efficacy. Relying on the theory of precision medicine, antibody-modified liposomes have become the next big field in active targeted liposomes — monoclonal antibodies, antibody Fab fragments, etc.
Mixing myeloma cells with B lymphocytes lets them split and make antibodies forever, and then the amazingly homogeneous screening and purification antibodies that are bound to a single antigen are monoclonal antibodies. They developed a nano-adapter that turns "cold tumors" into "hot tumors" through the stimulation of DNA damage and two immunological checkpoint inhibition mechanisms. Design delivers oxaliplatin and JQ1 to liposomes, and attaches T cell immunoglobulin mucin-3 monoclonal antibodies to the liposome's surface via metalloproteinase-2 (MMP-2). To do so, JQ1 blocks DNA repair and the PD-1 (programmed death receptor-1)/PD-L1 (programmed death ligand-1) pathway. This combined with the selective effect of monoclonal antibodies results in dual immune checkpoint inhibition and thus T cell activation.
Antibody Fab fragments are formed by antibodies under the action of proteolytic enzymes. They have the characteristics of small size and high penetration. They are not easily affected by immune cells such as macrophages and B cells in the body. Through their modification, liposomes can transport drugs to target sites. The researchers prepared a liposome (TFAb-Lip) coupled with an anti-tissue factor (TF) antibody Fab fragment. The liposome has a strong targeting effect on KLN205 squamous tumor cells and NIH3T3 fibroblasts expressing TF. At the same time, in vivo experiments in KLN205 solid tumor-bearing mice showed that TFAb-Lip not only aggregated in the tumor cell area, but was also highly enriched and widely distributed in the matrix area of the tumor, which can effectively inhibit the proliferation of tumor cells.
Due to some gene mutations in cancer cells, some receptors are overexpressed, while these receptors are less expressed or not expressed in normal cells. Based on the specific expression of tumors, the structure is modified with ligands or other functional groups through physical or chemical means to make the liposomes better play an active targeting role, which is conducive to drug delivery. Some researchers have designed a transferrin (Tf)-modified dihydroartemisinin and L-butionine-sulfoximine loaded liposomes and used them for targeted cancer treatment. The liposomes specifically recognize cancer cells through Tf-Tf receptor binding and are absorbed into the lysosomes of cancer cells, thereby destroying the redox balance in cancer cells and achieving the purpose of targeted treatment.
Nucleic acid aptamers are monomer DNA or RNA oligods that are between 25 and 90 nucleotide bases that are produced via artificial screening. Nucleic acid aptamers can create specialized three-dimensional spatial structures (secondary and tertiary structures), can recognise targets and act as target predators, and are highly specific and very affine. The key differences with aptamers compared to standard antibodies are that they're target rich, low-molecular-weight and programme controlled. They can help drugs get through tumours and spread evenly inside tumors. But they're also problematic because they are hard to develop and do not conform stable.
Thermoresponsive materials (dipalmitoylphosphatidylcholine, distearoylphosphatidylcholine, etc.) are a necessary part of thermosensitive liposomes. Temperature triggers drug release, and the higher the temperature, the more fluid the lipid membrane becomes from a hard colloidal state to an ultrapermeabilic liquid crystal state and the greater the diffusion of the encapsulated drug. Thermosensitive targeted liposomes are made up of very special lipid molecules, where the phase transition temperature is just a bit above body temperature. As the targeted zone gets heated (again, through an external heat source), the drug will also congregate there and become part of the targeted delivery process. In rhabdomyosarcoma, thermosensitive liposomes loaded with vinorelbine were delivered together with mild hyperthermia (39-43°C). This technique focuses more targeted drug to the tumour, and minimizes the accumulated drugs in the body that are off-target. Meanwhile, the targeting system is broadly generic and can be generalized to other chemotherapeutic agents.
In the pH-sensitive liposomes, pH-sensitive materials like dioleoylphosphatidylethanolamine were widely employed. If the local pH is slightly alkaline or acidic, then the carboxyl groups of fatty acids in these liposomes are protonated, so that the cell membrane and the liposomes fusion and drug delivery in cells is effective. When cancer cells produce a lot of lactic acid from glycolysis, which in turn makes the tumor tissue environment acidic, they use this to engineer the liposomes for a targeting effect. Unlike in the case of the free drug delivery, the targeted liposomes inhibit the tumors much better. The liposomes then use the conversion mediation provided by the polar head of dioleyl phosphatidyl amide to collapse the cell's membrane in the presence of acid, bind the liposomes to the endosome membrane, and thus send the drugs into the cytoplasm.
Prepared based on the high ROS concentration in tumor lesions. On the one hand, ROS can directly react with proteins, transcription factors, etc. to regulate the conduction pathway; on the other hand, ROS can oxidatively modify biological molecules and change their functions. ROS-sensitive liposomes modify their lipids so that after reaching the lesion area, the lipid membrane structure is changed under the oxidative action of ROS, thereby releasing the encapsulated drugs.
Breast cancer is the most common clinical malignant tumour, its incidence rate has outpaced lung cancer and is the highest rate of cancer in women worldwide. Chemotherapy now has an unquestionable place in the treatment of breast cancer but chemo drugs are likely to cause oxidative stress reaction in the tumor tissues and result in patients' toxic and adverse effects (eg, weakening immunity, suppression of bone marrow and toxicity of the heart). Thus, nano-drug targeted systems to drugs have become a field of study and a fever. Among them, liposomes, as good drug carriers, can well protect drugs from the influence of the external tumor environment, and reduce drug side effects on the basis of giving full play to their efficacy. In order to improve the circulation and targeting ability of drugs in the body, researchers have developed a functional liposome loaded with taxane analogs (LTX) modified with nucleotide-lipid derivatives for the treatment of drug-resistant breast cancer. GT28nt is a specific ligand for nucleolin receptors overexpressed on drug-resistant breast cancer. Liposomes modified with this derivative have extremely strong targeting properties. Encapsulating LTX in liposomes can solve the problem that LTX itself is difficult to use in clinical practice due to its poor water solubility, and overcome the multidrug resistance caused by breast cancer. In addition, the researcher also labeled the functional liposome GT28NT-FDL with the fluorescent probe DiR and found that its accumulation in tumor tissue was much higher than that of free DiR; in the blood, the fluorescence signal of GT28nt-FDL could remain unchanged for 48 hours, while the free DiR disappeared rapidly, proving that the liposome has a strong circulation effect in the blood.
Lung cancer is a common malignant growth. Lesser causes of lung cancer are smoking and pollution. It can be treated surgically. But if tumor edges and lymph node locations are not completely removed, they could re-metastasis, and in clinical practice chemo is the treatment of choice. Given the complexity of the tumor microenvironment and the injury to healthy tissue caused by chemo, a tumour-targeted drug delivery system must be designed fast and safely. Liposomes can mash with extracellular particles to be more targeted and biocompatible. Researchers combined liposomes with exosomes made by chimeric antigen receptor-T (CAR-T) cells to create a new kind of hybrid nanovesicle, Lip-CExo@PTX, for the treatment of lung cancer. Liposomes in this process solved the issue of exosomes' low drug loading capacity. By their very nature, cytotoxic particles (granzyme B and perforin) and PTX were packed within liposomes and between lipid bilayers, respectively. A large percentage of Lip-CExo@PTX has been successfully tested in experiments and it's shown to be able to remain localized and biocompatible in lung tissue upon intravenous injection at 95 percent lipoconcentration. Here liposomes fully leverage the CAR-T exosomes' potential to bind mesothelin (MSLN) and PD-L1 and suppress PD-L1 in the tumor and deliver the drug to MSLN-positive tumours, so T cells don't become exhausted and it has more powerful anti-tumor activity. Liposomes can be formulated as inhalable liposomes as well for intravenous infusion or oral administration.
References
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.
1. Download the template.
2. Enter product information on the template (maximum number of products: 200).
3. Load the file using selector below.